filmov
tv
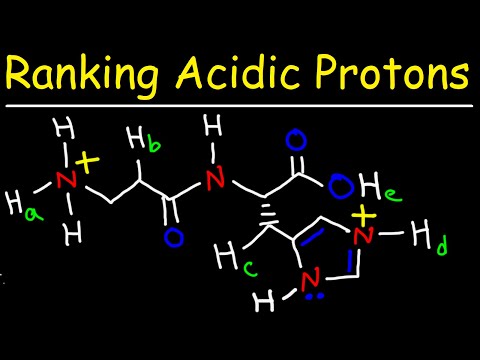
Comparing Acidity using ARIO

Показать описание
This video was created for a first semester sophomore organic chemistry course that uses chapters 1-10 in David Klein's organic chemistry textbook. If you use a different textbook, you will probably still find these useful, but if you notice any differences you should consult with your instructor.
Comparing Acidity using ARIO
Acids & Bases - Inductive Effect, Electronegativity, Hybridization, Resonance & Atomic Size
Comparing Acidity using ARIO v2
3.2 Ranking Acids and Bases | Organic Chemistry
Ranking Acidity, Using pKa, and Drawing Arrows in Acid-Base Reactions
30a: Ranking acids and bases by strength
Using ARIO
Using ARIO to solve acidity problems
Ranking Protons in order of Increasing Acidity Using pKa Values
Determining Acidity Using Qualitative Perspective- ARIO
25: Practice ranking molecules in order of acidity
Inductive Effect - Acids and Bases
How to Predict Acid Base Reactions using ARIO
Acidity 1 - ARIO
Acidity Qualitative ARIO
Acid-base- ARIO 12, Predicting the position of the equilibrium- Dr. Tania CS
Using ARIO to solve acidity problems v2
Acid-base-ARIO 6, determining the most acidic hydrogen in a molecule- Dr. Tania CS
ARIO--Determining Position of Equilibria | CHEM 123 @ UBC
ARIO Organic Chemistry Practice Problems; Step by Step Walkthrough on Ranking Stability of Protons
Acidity 3 - Finishing Up ARIO (Check description for correction)
Lec 6 Comparing Acid Strengths Using ARIO | Chapter 3 (Part 2 of 2)
Comparing Acidity with pKas
OZZTUBE 19: Qualitation of Acidity Using ARIO and OZZ's Big 4
Комментарии
 0:12:54
0:12:54
 0:09:19
0:09:19
 0:12:54
0:12:54
 0:35:47
0:35:47
 0:31:26
0:31:26
 0:11:38
0:11:38
 0:03:55
0:03:55
 0:05:49
0:05:49
 0:05:20
0:05:20
 0:13:17
0:13:17
 0:08:23
0:08:23
 0:11:20
0:11:20
 0:11:50
0:11:50
 0:13:48
0:13:48
 0:13:01
0:13:01
 0:06:54
0:06:54
 0:05:49
0:05:49
 0:05:24
0:05:24
 0:07:14
0:07:14
 0:19:57
0:19:57
 0:09:59
0:09:59
 0:20:57
0:20:57
 0:07:46
0:07:46
 0:29:17
0:29:17