filmov
tv
How to calculate Electronegativity

Показать описание
A tutorial on how to use electronegativity values to classify bonds.
How to Calculate Electronegativity
How to calculate Electronegativity? Easy Trick
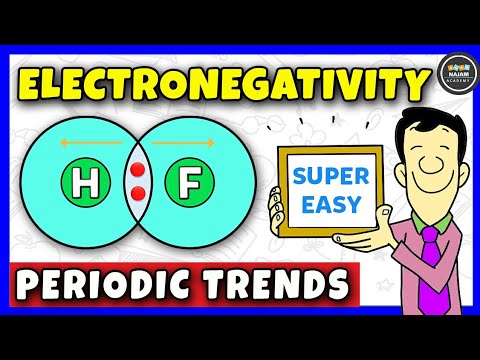
Electronegativity, Basic Introduction, Periodic Trends - Which Element Is More Electronegative?
How to calculate electronegativity (EN)
Electronegativity
The Chemical Bond: Covalent vs. Ionic and Polar vs. Nonpolar
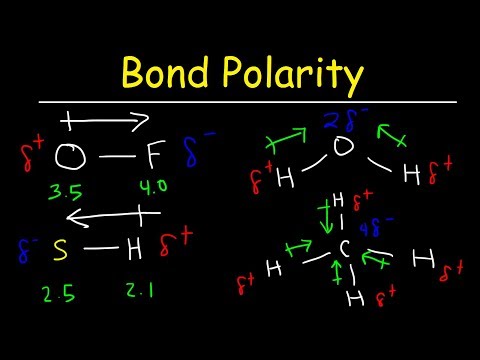
Bond Polarity, Electronegativity and Dipole Moment - Chemistry Practice Problems
Short trick to Learn Electronegativity Values 🧐🧐💪🏻⚡| Motion NEET | #neet #shorts #poonammam #tricks...
Electronegativity
The Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity
How to calculate electronegativity value?
How to Find Electronegativity and Electronegativity Differences?
HOW TO CALCULATE ELECTRONEGATIVITY DIFFERENCE
Formula’s of Electronegativity #viral #chemistry #ytshorts
Periodic Trends: Electronegativity, Ionization Energy, Atomic Radius - TUTOR HOTLINE
Electronegativity | Periodic Trends | Chemistry
Electronegativity Grade 11 Chemistry Polarity of Bonds
Pauling, Mulliken and Allred Rochow scale of electronegativity. Application of electronegativity.
How to calculate Electronegativity
Best 💯TRICK 🔥💡to learn ELECTRONEGATIVITY values #jee #iitjee #jeemains #iit #tricks #trick #neet...
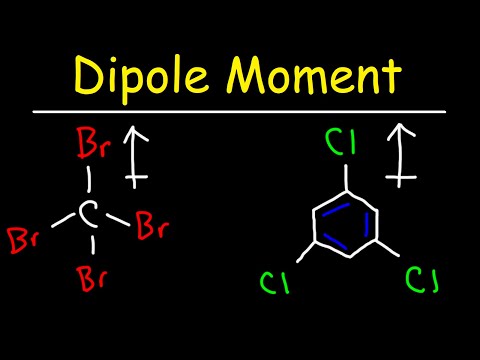
Dipole Moment, Vectors, & Electronegativity - Organic Chemistry
Polar Molecules Tutorial: How to determine polarity in a molecule
How to calculate electronegativity?
11 chap 3 | Periodic Table 07||Electronegativity IIT JEE || Electronegativity NEET ||
Комментарии
 0:01:54
0:01:54
 0:06:30
0:06:30
 0:11:42
0:11:42
 0:01:50
0:01:50
 0:02:12
0:02:12
 0:03:33
0:03:33
 0:11:21
0:11:21
 0:01:00
0:01:00
 0:01:47
0:01:47
 0:07:53
0:07:53
 0:01:29
0:01:29
 0:10:03
0:10:03
 0:10:00
0:10:00
 0:00:29
0:00:29
 0:24:55
0:24:55
 0:10:00
0:10:00
 0:16:15
0:16:15
 0:18:16
0:18:16
 0:08:55
0:08:55
 0:03:03
0:03:03
 0:05:24
0:05:24
 0:10:36
0:10:36
 0:01:19
0:01:19
 0:42:19
0:42:19