filmov
tv
O3 Resonance Structures (Ozone)

Показать описание
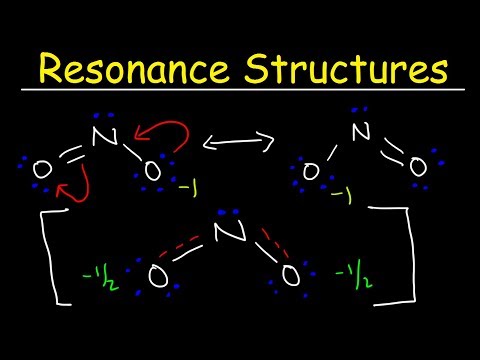
There are two resonance structures for O3 (ozone).
- Resonance structures are necessary to show how electrons are distributed in chemical bonds in a molecule.
- Remember, the molecule isn't flipping back and forth between structures! It's an average of the resonance structures.
- The double arrow symbol drawn between resonance structures does not mean equilibrium or any sort of change. It only shows that there is more than one way to draw the structure. It's not a very good choice of symbols, really.
- Resonance structures are necessary to show how electrons are distributed in chemical bonds in a molecule.
- Remember, the molecule isn't flipping back and forth between structures! It's an average of the resonance structures.
- The double arrow symbol drawn between resonance structures does not mean equilibrium or any sort of change. It only shows that there is more than one way to draw the structure. It's not a very good choice of symbols, really.
O3 Resonance Structures (Ozone)
Resonance Structures of O3, Ozone
Resonance structures of ozone molecule/resonance hybrid of ozone/ozone resonance l
O3 Lewis Structure - Ozone
Resonance structure of ozone l ozone Resonance structure l ozone formula and structure l chemistry
Resonance structures of Ozone (O3)
Draw the resonating structure of: Ozone molecule
Ozone Gas - Resonance Structure Animation
Ozone Resonance Structures
Calculating O3 Formal Charges: Calculating Formal Charges for O3 (Ozone)
Lewis Dot Structure for O3 (Ozone)
Electron Dot Structure of Ozone 👍👍✍️✍️
Resonance - O3 ozone example
O3 Lewis structure- How to draw dot structure of Ozone?#jeeadvanced
Lewis structure of ozone #chemistry#shorts#lewisdotstructure#inorganicchemistry#neetchemistry
Ozone Lewis Structure: How to Draw the Lewis Structure for Ozone
Resonance Structures, Basic Introduction - How To Draw The Resonance Hybrid, Chemistry
Bonding 36: The Resonance Structures of Ozone
Draw a Lewis structure for the ozone molecule, O3, and also the resonance structure.
O3 Lewis Structure Ozone
Lewis Structure of Ozone
Draw the resonating structure of (i) Ozone molecule (ii) Nitrate ion
Resonance Structures - Introduction for Beginners
O3 Lewis Structure (Ozone)
Комментарии
 0:01:26
0:01:26
 0:05:05
0:05:05
 0:03:05
0:03:05
 0:05:50
0:05:50
 0:00:59
0:00:59
 0:05:38
0:05:38
 0:05:04
0:05:04
 0:01:16
0:01:16
 0:01:01
0:01:01
 0:01:45
0:01:45
 0:02:25
0:02:25
 0:00:16
0:00:16
 0:20:26
0:20:26
 0:00:58
0:00:58
 0:01:00
0:01:00
 0:01:02
0:01:02
 0:10:31
0:10:31
 0:04:53
0:04:53
 0:00:57
0:00:57
 0:06:38
0:06:38
 0:01:37
0:01:37
 0:03:08
0:03:08
 0:06:54
0:06:54
 0:02:13
0:02:13