filmov
tv
Free Radical Substitution, Alkane Mechanism - Organic Chem

Показать описание
In this video we want to discuss Free Radical Substitution for Alkanes.
Understanding the free radical substitution mechanism is important for A Level Chemistry, and we need to describe the mechanism in detail using curly arrows.
For this discussion we are using the chlorination of methane. For bromination of methane we simply replace the chlorine with bromine element.
The first step of free radical substitution is initiation step, where the chlorine to chlorine bond undergoes homolytic fission to form 2 chlorine atoms or radicals.
The radicals are extremely reactive and kickstarts the reaction, hence we call this the initiation step.
Second step is propagation step, where the radical attacks a stable molecule and generates a stable molecule and another radical. The total number of radicals stay the same which causes the reaction to propagate, hence the name propagation.
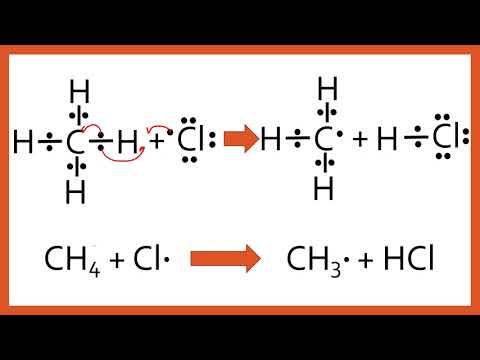
First propagation step is where the chlorine radical attacks methane, breaks the C-H bond homolytically and forms HCl to generate a methyl radical.
Second propagation step is where the methyl radical attacks Cl2 molecule, breaks the Cl-Cl bond homolytically and forms CH3Cl or chloromethane and generate a chlorine radical.
Take note 2 propagation steps are required for one substitution.
So for this example, since we are doing a disubstitution, we need 2 more propagation steps for the second substitution to form dichloromethane.
Also, it is important to note that free radical substitution is a totally random process, so the mechanism that we are describing is not the only steps that are taking place, but the shortest pathway to reach the desired product that is requested in the question.
Once we form the required product we can move on to the termination step.
Finally, last step will be termination step where any two radicals will undergo homolytic fusion and form a stable product.
An interesting product from termination step is the formation of ethane, where the carbon number is double that of starting alkane, methane in this case.
For the detailed discussion on how to draw the mechanism, check out this video!
Topic: Alkanes (Hydrocarbon), Organic Chemistry, A Level Chemistry, Singapore
Found this video useful?
Please LIKE this video and SHARE it with your friends!
Any feedback, comments or questions to clarify?
Suggestions for new video lessons?
Drop them in the COMMENTS Section, I would love to hear from you!
Need an experienced A Level Chemistry tutor to boost your grades?
Check out the SEVEN reasons why Chemistry Guru can provide the best A Level Chemistry Tuition for you:
-~-~~-~~~-~~-~-
Please watch my latest video: "2019 P1 Q2 - Deflection of Charged Particle in Electric Field"
-~-~~-~~~-~~-~-
Understanding the free radical substitution mechanism is important for A Level Chemistry, and we need to describe the mechanism in detail using curly arrows.
For this discussion we are using the chlorination of methane. For bromination of methane we simply replace the chlorine with bromine element.
The first step of free radical substitution is initiation step, where the chlorine to chlorine bond undergoes homolytic fission to form 2 chlorine atoms or radicals.
The radicals are extremely reactive and kickstarts the reaction, hence we call this the initiation step.
Second step is propagation step, where the radical attacks a stable molecule and generates a stable molecule and another radical. The total number of radicals stay the same which causes the reaction to propagate, hence the name propagation.
First propagation step is where the chlorine radical attacks methane, breaks the C-H bond homolytically and forms HCl to generate a methyl radical.
Second propagation step is where the methyl radical attacks Cl2 molecule, breaks the Cl-Cl bond homolytically and forms CH3Cl or chloromethane and generate a chlorine radical.
Take note 2 propagation steps are required for one substitution.
So for this example, since we are doing a disubstitution, we need 2 more propagation steps for the second substitution to form dichloromethane.
Also, it is important to note that free radical substitution is a totally random process, so the mechanism that we are describing is not the only steps that are taking place, but the shortest pathway to reach the desired product that is requested in the question.
Once we form the required product we can move on to the termination step.
Finally, last step will be termination step where any two radicals will undergo homolytic fusion and form a stable product.
An interesting product from termination step is the formation of ethane, where the carbon number is double that of starting alkane, methane in this case.
For the detailed discussion on how to draw the mechanism, check out this video!
Topic: Alkanes (Hydrocarbon), Organic Chemistry, A Level Chemistry, Singapore
Found this video useful?
Please LIKE this video and SHARE it with your friends!
Any feedback, comments or questions to clarify?
Suggestions for new video lessons?
Drop them in the COMMENTS Section, I would love to hear from you!
Need an experienced A Level Chemistry tutor to boost your grades?
Check out the SEVEN reasons why Chemistry Guru can provide the best A Level Chemistry Tuition for you:
-~-~~-~~~-~~-~-
Please watch my latest video: "2019 P1 Q2 - Deflection of Charged Particle in Electric Field"
-~-~~-~~~-~~-~-
Комментарии
 0:12:29
0:12:29
 0:13:45
0:13:45
 0:30:19
0:30:19
 0:09:53
0:09:53
 0:03:35
0:03:35
 0:17:29
0:17:29
 0:01:59
0:01:59
 0:12:24
0:12:24
 1:56:22
1:56:22
 0:04:01
0:04:01
 0:08:03
0:08:03
 0:08:14
0:08:14
 0:09:12
0:09:12
 0:35:20
0:35:20
 0:12:40
0:12:40
 0:34:45
0:34:45
 0:10:01
0:10:01
 0:17:16
0:17:16
 0:14:13
0:14:13
 0:10:20
0:10:20
 0:04:07
0:04:07
 0:09:52
0:09:52
 0:05:03
0:05:03
 0:01:43
0:01:43