filmov
tv
Atomic Species Balances

Показать описание
Atomic Species Balances
Lecture 23 Atomic Species Balance on Reactive Systems
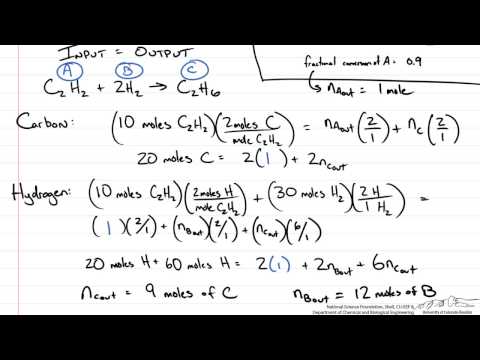
Example Atomic Species Balance (pt 11)
Molecular and atomic species balance
Atomic Balances - Example
3.2 Balance for atomic species
Three Methods for Reactive MEB Problems
Atomic Species Balances with excel
MBReactive Part 8 - Atomic Species Balance Method
Atomic Species
Chapter 4.7 Molecular Species Balance VS. Atomic Species Balance on Reactive Process
Molecular and atomic species balance: Example 8
Molecular Species Balances
Molecular Species Balance vs Atomic Species Balance (Principle of Chemical Processes)
Example 11: Combustion reaction (Atomic species balance)
Analysis of Atomic species balance and extend of reaction method
Reactive process - atomic species balance
Reactions with Atomic Balances
MBReactive Part 10 - Degree of Freedom (Molecular, Atomic and Extent of Reaction Methods)
Fundamentals ll Atomic balance ~ part 1
Three Methods for Balancing Reactive Processes
Atomic Species Material Balances
Analysis of atomic species balance and extent of reactions method (KNC 1503 Assignment 2)
Lecture 25 Material balance on Reactive Systems with Multiple Reactions
Комментарии
 0:06:56
0:06:56
 0:15:45
0:15:45
 0:05:32
0:05:32
 0:08:47
0:08:47
 0:14:03
0:14:03
 0:05:04
0:05:04
 0:10:45
0:10:45
 0:07:08
0:07:08
 0:14:09
0:14:09
 0:06:53
0:06:53
 0:09:29
0:09:29
 0:28:51
0:28:51
 0:04:20
0:04:20
 0:19:21
0:19:21
 0:19:04
0:19:04
 0:17:31
0:17:31
 0:23:20
0:23:20
 0:11:20
0:11:20
 0:10:37
0:10:37
 0:06:30
0:06:30
 0:15:08
0:15:08
 0:27:24
0:27:24
 0:06:36
0:06:36
 0:21:38
0:21:38