filmov
tv
Writing Condensed/Abbreviated Electron Configuration for Carbon (C)

Показать описание
To write condensed electron configurations (also called abbreviated electron configurations) Carbon (C) we first write the full electron configuration for the element. Once we have the full configuration we put brackets around the config for the Noble Gas core. We can then replace this with the element symbol for the Noble Gas, leaving the rest of the configuration visible (these are the valence electrons).
For example, for Carbon, atomic number 6 on the Periodic Table:
Carbon 1s2 2s2 2p2 (full)
Carbon [1s2] 2s2 2p2 (Noble Gas in brackets)
Carbon [He] 2s2 2p2 (Condensed config)
For help writing electron configurations for elements:
The condensed electron configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. It places an emphasis on the valence electrons (the electrons written after the Noble Gas core). This makes it easier to understand and predict how atoms will interact to form chemical bonds.
For example, for Carbon, atomic number 6 on the Periodic Table:
Carbon 1s2 2s2 2p2 (full)
Carbon [1s2] 2s2 2p2 (Noble Gas in brackets)
Carbon [He] 2s2 2p2 (Condensed config)
For help writing electron configurations for elements:
The condensed electron configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. It places an emphasis on the valence electrons (the electrons written after the Noble Gas core). This makes it easier to understand and predict how atoms will interact to form chemical bonds.
Writing Condensed/Abbreviated Electron Configurations
Writing Condensed/Abbreviated Electron Configuration for Aluminum (Al)
Writing Condensed/Abbreviated Electron Configuration for Carbon (C)
Writing Condensed/Abbreviated Electron Configuration for Chlorine (Cl)
How to Write the Electron Configuration for an Element in Each Block
Electron Configuration With Noble Gas Notation
Writing Abbreviated Electron Configurations
Abbreviated Electron Configuration
Electron Configuration - Basic introduction
Abbreviated electron configuration of Si
How to write the Electron Configuration for an element in each block - Dr K
How to Write the Electron Configuration of an Element | Study Chemistry With Us
A Level Chemistry Revision 'Shorthand Electron Configuration'
Electronic Configuration - Transition Metals
Orbital Diagrams and Electron Configuration - Basic Introduction - Chemistry Practice Problems
Condensed Electron Configurations
Noble Gas/Abbreviated Electron Configurations
Full and Abbreviated Electron Configuration of Tin Sn
Writing Abbreviated Electron Configurations. (Chemistry Ch. 3, Part 6)
Writing Abbreviated (Noble Gas) Electron Configurations
07 - Abbreviated Electron Configurations
Write a noble gas or shorthand electron configuration arsenic
Using the Diagonal Rule to Write Electron Configurations
Full and Abbreviated Electron Configuration of Arsenic As
Комментарии
 0:06:29
0:06:29
 0:01:19
0:01:19
 0:01:30
0:01:30
 0:01:23
0:01:23
 0:07:23
0:07:23
 0:10:06
0:10:06
 0:07:15
0:07:15
 0:05:54
0:05:54
 0:10:19
0:10:19
 0:01:22
0:01:22
 0:09:53
0:09:53
 0:30:45
0:30:45
 0:04:07
0:04:07
 0:04:14
0:04:14
 0:12:12
0:12:12
 0:03:34
0:03:34
 0:05:21
0:05:21
 0:00:28
0:00:28
 0:12:02
0:12:02
 0:01:24
0:01:24
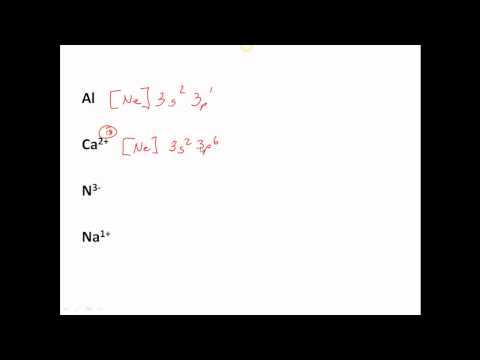 0:06:59
0:06:59
 0:02:15
0:02:15
 0:01:59
0:01:59
 0:00:28
0:00:28