filmov
tv
Late Night Chemistry: Cobalt(I)

Показать описание
Late on a cool summer night in my countryside lab, I prepare a rather interesting and rather stinky complex containing the elusive Cobalt(I).
I'm not responsible for damage to persons or property caused by the replication of this experiment.
I'm not responsible for damage to persons or property caused by the replication of this experiment.
Late Night Chemistry: Cobalt(I)
Unlocking the Secrets of Hydrogen, Carbon, Silicone, and Cobalt Spectrometry
Colorful Cobalt (Reaction of Cobalt carbonate with Hydrochloric acid) [ReUpl]
Discovering the Elemental Bond The Vortex of Hydrogen, Carbon, Silicon, Cobalt, and Rhodium
Late Night Chemistry: Vanillyl Alcohol
This chemical reaction looks like magic (iodine clock)
Diane Morgan: Philomena Cunk Would Love To Interview Elon Musk
POV: you’re 6’9” 400 pounds and booked the middle seat
Colorful Complexes Ep 4: Sodium-Potassium Hexanitrocobaltate
Growing up Pentecostal... #short
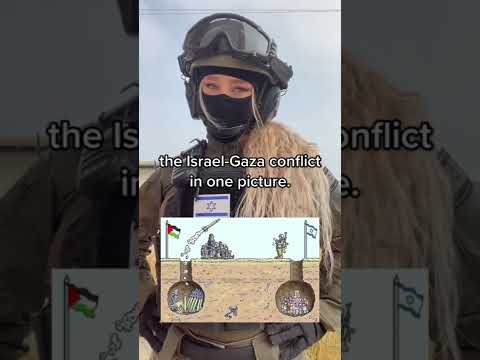
The Israel-Gaza conflict in one picture… #Israel #gaza #idf
'Iron Wine' (& other complexes of Nitroguanidine)
Elon Musk's first wife describes their relationship
Telaraña con slime?🕷🕸 #hombrearaña #spiderman #spideverse #blog #shorts
Chemistry Seminar: UWI Mona knowledge applied with cobalt- and vanadium-containing compounds | 2021
Preparation of Cesium Tetrachloroiodate
Turning lead into gold (lead iodide)
The Prof watches Breaking Bad (at last)
Elements: Silver
Do NOT buy these perfumes!!
This Is What Scientists Found at the Bottom of the Niagara Falls That Left Them so Disturbed
Making Chevreul's Salt
Quick Inorganic Preparations Episode II
KICl4 prep
Комментарии
 0:10:13
0:10:13
 0:00:26
0:00:26
 0:03:59
0:03:59
 0:00:49
0:00:49
 0:09:07
0:09:07
 0:02:02
0:02:02
 0:08:13
0:08:13
 0:00:18
0:00:18
 0:07:42
0:07:42
 0:00:15
0:00:15
 0:00:08
0:00:08
 0:12:19
0:12:19
 0:08:18
0:08:18
 0:00:14
0:00:14
 0:58:45
0:58:45
 0:07:35
0:07:35
 0:01:46
0:01:46
 0:21:39
0:21:39
 0:04:57
0:04:57
 0:00:51
0:00:51
 0:05:42
0:05:42
 0:06:00
0:06:00
 0:05:49
0:05:49
 0:04:59
0:04:59