filmov
tv
Making esters - Part 1 | Chemistry Tutorial

Показать описание
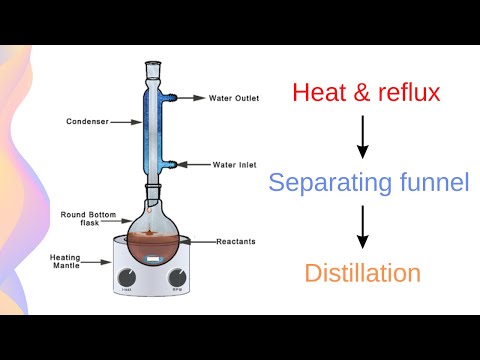
Chemistry lecturer from the School of Molecular & Life Sciences, Dr Alexandra Yeung takes us through an experiment to create esters, or ethyl ethanoate.
Making esters - Part 1 | Chemistry Tutorial
IGCSE Lab: Making esters
Making esters - Part 2 | Chemistry Tutorial
GCSE Chemistry - Esters
Making esters!! #chemistry #organicchemistry #experiment
Esters and Making Esters
Esterification test | #laboratory | formation of ester #ester @pharmacists kgi Wale #bpharma
Grade 12 Practical Esters Part 1 of 3
MIND-BLOWING General Organic Chemistry Secrets Revealed
Synthesis of Esters
Esterification--Making Esters from Carboxylic Acids
Making esters (GCSE/A-level Chemistry)
TO STUDY ESTERIFICATION REACTION BETWEEN ALCOHOL AND CARBOXYLIC ACID
Esterification Synthesis Lab - Banana, Wintergreen, Flowers
Making esters | A-level Chemistry | Year 2
Formation of Ester (Chemistry Lab Experiment)
Esterification: Reflux, Isolation and Purification // HSC Chemistry
Making Esters | Nucleophilic Addition-Elimination Mechanism #alevelchemistry #revision
Carbon compound | Esters | Part 1
4.8 - SCH4C Making Esters
10.3 Carboxylic Acids and Esters part 1
3 ESSENTIAL Terrarium Tips For Beginners!
Did you know this about Stray? 🙀
Naming Organic Molecules Grade 12 | Carboxylic Acids, Esters
Комментарии
 0:13:51
0:13:51
 0:10:31
0:10:31
 0:05:00
0:05:00
 0:02:02
0:02:02
 0:00:29
0:00:29
 0:00:04
0:00:04
 0:00:23
0:00:23
 0:01:04
0:01:04
 1:12:46
1:12:46
 0:04:25
0:04:25
 0:06:52
0:06:52
 0:04:37
0:04:37
 0:03:19
0:03:19
 0:05:23
0:05:23
 0:23:22
0:23:22
 0:00:25
0:00:25
 0:09:22
0:09:22
 0:00:44
0:00:44
 0:04:52
0:04:52
 0:05:01
0:05:01
 0:58:28
0:58:28
 0:00:28
0:00:28
 0:00:37
0:00:37
 0:06:21
0:06:21