filmov
tv
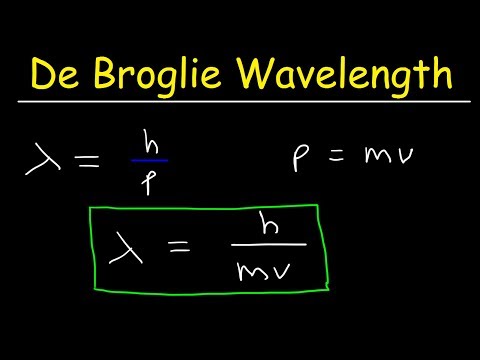
Calculation of de Broglie wavelength of the electron

Показать описание
An electron microscope uses an electron beam of energy E=1.0 keV. Can this microscope be used to obtain the image of an individual atom? (The size of an atom ~10^{−10} m.)
De Broglie Wavelength Problems In Chemistry
De Broglie wavelength | Physics | Khan Academy
Calculation of de Broglie wavelength of the electron
⚗️ Solve De Broglie Wavelength Problems
Your Daily Equation #9: De Broglie Wavelength
How to solve a de broglie wavelength problem
Finding Wavelength, Frequency, Velocity Using de Broglie's Equation | Study Chemistry With Us
De Broglie wavelength calculation
De Broglie Hypothesis | De Broglie Wavelength
Derive De-Broglie Wave Equation | Quantum Chemistry | Physical Chemistry
Calculate the de-Broglie wavelength of an electron of kinetic energy 100 eV
Deriving The De Broglie Wavelength
Calculate the de Broglie Wavelength (λ) From Mass and Velocity 001
de broglie's wavelength calculation simple problem (electron in accelerated potential)
de Broglie’s proposal
The De Broglie Wavelength Equation
The de Broglie Wavelength
Comparing de Broglie wavelengths: Solved example | Dual nature of light | Physics | Khan Academy
Physics CH 0.5: Standard Units (36 of 41) The de Broglie Wavelength
Calculate the de Broglie wavelength associated with a proton, moving with a velocity c/20.
deBroglie Wavelength Calculation Example (velocity given wavelength)
How to find de-broglie wavelength I De Broglie wavelength problems I
de Broglie wavelength in different frames
Calculate de-Broglie wavelength when
Комментарии
 0:11:21
0:11:21
 0:11:20
0:11:20
 0:03:07
0:03:07
 0:05:21
0:05:21
 0:21:29
0:21:29
 0:02:37
0:02:37
 0:33:23
0:33:23
 0:05:12
0:05:12
 0:09:05
0:09:05
 0:01:43
0:01:43
 0:02:39
0:02:39
 0:03:41
0:03:41
 0:06:04
0:06:04
 0:03:30
0:03:30
 0:10:37
0:10:37
 0:02:59
0:02:59
 0:02:06
0:02:06
 0:11:40
0:11:40
 0:04:45
0:04:45
 0:07:32
0:07:32
 0:04:18
0:04:18
 0:10:44
0:10:44
 0:14:53
0:14:53
 0:01:41
0:01:41