filmov
tv
Electrophilic Substitution of Pyrrole and Pyridine
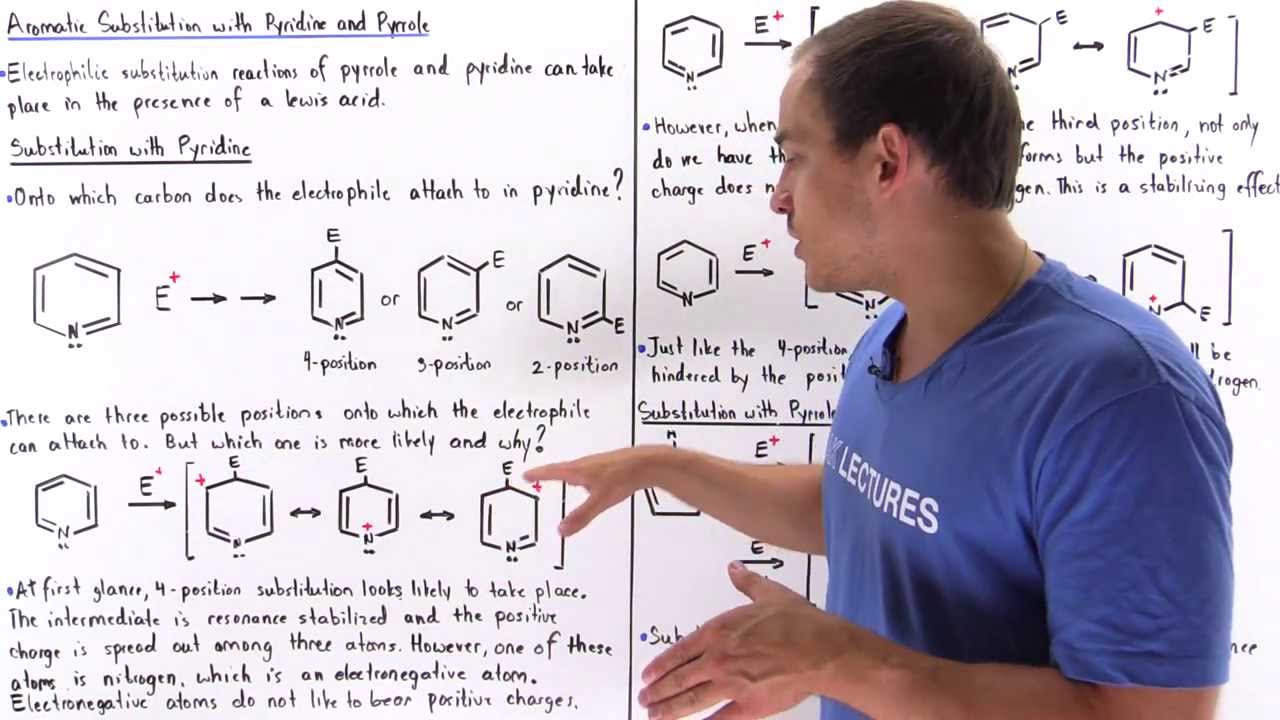
Показать описание
Electrophilic Substitution of Pyrrole
Electrophilic Substitution of Pyrrole and Pyridine
Aromatic Compounds & Heterocycles - Nucleophilic & Electrophilic Aromatic Substitution React...
Orientation of electrophilic substitution in pyrrole
Electrophilic substitution reaction in pyrrole
EAS with Heterocyclopentadienes (Pyrrole, Furan, Thiophene)
ELECTROPHILIC SUBSTITUTION IN FURAN, PYRROLE & THIOPHENE
Chemical Reactions of Pyrrole
Lecture- 1246Topic- PROPERTIES AND STRUCTURE OF PYRIDINE
9 Electrophilic Substitution & Reduction of Pyrrole
Heterocycles Part 1: Furan, Thiophene, and Pyrrole
Pyrrole: Electrophilic Substitution Reactions Lecture 1
Electrophilic substitution in Furan, Pyrrole and Thiophene
Orientation and electrophilic substitutions reaction of pyrrole《 part 1》
L-3 Heterocyclic compounds || Pyrrole || Electrophilic substitution reaction
Electrophilic Substitution Reaction of Pyrrole & It's mechanism || Organic Chemistry, B.Sc.
The ELECTROPHILIC SUBSTITUTION REACTION in PYRROLE.
Why does Pyridine undergo Electrophilic Aromatic Substitution under vigorous conditions ?
EAS with Pyrrole
Electrophilic Substitution in PYRROLE,THIOPHENE and FURAN
Pyrrole: Electrophilic Substitution Reactions Lecture 2
I L 5 I Heterocyclic Compounds I Mechanism & Orientation of Electrophilic Substitution Reaction
Electrophilic substitution in pyridine..
ELECTROPHILIC SUBSTITUTION REACTION OF PYRROLE AND ACIDIC BEHAVIOUR OF PYRROLE
Комментарии
 0:06:01
0:06:01
 0:06:38
0:06:38
 0:31:28
0:31:28
 0:09:20
0:09:20
 0:06:05
0:06:05
 0:14:02
0:14:02
 0:01:05
0:01:05
 0:08:33
0:08:33
 0:17:31
0:17:31
 0:05:30
0:05:30
 0:07:30
0:07:30
 0:16:46
0:16:46
 0:03:59
0:03:59
 0:42:13
0:42:13
 0:08:53
0:08:53
 0:11:59
0:11:59
 0:00:15
0:00:15
 0:03:28
0:03:28
 0:14:36
0:14:36
 0:00:39
0:00:39
 0:17:50
0:17:50
 0:14:21
0:14:21
 0:04:32
0:04:32
 0:19:55
0:19:55