filmov
tv
How to Balance KMnO4 + Fe = FeO + K2O + MnO2

Показать описание
In this video we'll balance the equation KMnO4 + Fe = FeO + K2O + MnO2 and provide the correct coefficients for each compound.
To balance KMnO4 + Fe = FeO + K2O + MnO2 you'll need to be sure to count all of atoms on each side of the chemical equation.
Once you know how many of each type of atom you can only change the coefficients (the numbers in front of atoms or compounds) to balance the equation for Potassium permanganate + Iron.
Important tips for balancing chemical equations:
Only change the numbers in front of compounds (the coefficients).
Never change the numbers after atoms (the subscripts).
The number of each atom on both sides of the equation must be the same for the equation to be balanced.
For a complete tutorial on balancing all types of chemical equations, watch my video:
Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
To balance KMnO4 + Fe = FeO + K2O + MnO2 you'll need to be sure to count all of atoms on each side of the chemical equation.
Once you know how many of each type of atom you can only change the coefficients (the numbers in front of atoms or compounds) to balance the equation for Potassium permanganate + Iron.
Important tips for balancing chemical equations:
Only change the numbers in front of compounds (the coefficients).
Never change the numbers after atoms (the subscripts).
The number of each atom on both sides of the equation must be the same for the equation to be balanced.
For a complete tutorial on balancing all types of chemical equations, watch my video:
Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
How to Balance KMnO4 + HCl = KCl + MnCl2 + H2O + Cl2
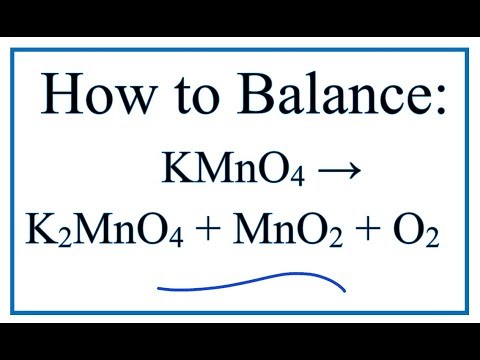
How to Balance KMnO4 + heat = K2MnO4 + MnO2 + O2 (Decomposition of Potassium permanganate)
Easy Method For Prepration of KMnO4 ,Tricks #neet #jee #chemistry #dblock #shorts #insightclasses
How to Balance KMnO4+HCl → KCl+MnCl2+Cl2+H2O
How to Balance KMnO4+HCl → KCl+MnCl2+Cl2+H2O
How to balance KMnO4 + HCl = KCl + MnCl2 + H2O + Cl2
Trick to remember Preparation of KMnO4👍|ASN CHEMISTRY
KMnO4 + HCl balanced equation
How to Balance KMnO4 + Fe = FeO + K2O + MnO2
How to balance KMnO4 = K2MnO4 + MnO2 + O2
KMnO4 / redox reactions / d & f block elements / class 12
Mind Blowing Science Reaction of H2O2 and KMNO4 🤯🔥 #chemistry #reaction #scienceexperiment #science...
Kmno4 reaction with H2so4.
Equation for KMnO4 + H2O (Potassium Permanganate + Water)
How to Balance Redox Reaction | Oxidation Number Method| KMnO4+H2C2O4+H2SO4=K2SO4+MnSO4+CO2+H2O
Best way to explain and remember KMnO4 reactions by suresh sir / class 12
CHEMICAL BALANCE OF KMNO4+H2SO4=K2SO4+MNSO4+H2O+O | CHEMICAL BALANCE
HOW TO BALANCE KMNO4 + HCL GIVES KCL+MNCL2+H2O+CL2
How to Write the Net Ionic Equation for H2O2 + KMnO4 + H2SO4
KMnO4 to conc. H2SO4 , Explosive solution
KMnO4+H2SO4=K2SO4+MnSO4+H2O+O2 balance the chemical equation @mydocumentary838
Oxidation number of Mn in KMnO4 #shorts #youtubeshorts #neet
Tricks to balance oxidising reactions of Potassium Pemanganate (KMnO4)
How to Balance the H2S+KMnO4=MnO2+H2SO4+K Chemical Equation
Комментарии
 0:02:27
0:02:27
 0:01:27
0:01:27
 0:00:27
0:00:27
 0:03:16
0:03:16
 0:06:00
0:06:00
 0:03:12
0:03:12
 0:02:36
0:02:36
 0:03:46
0:03:46
 0:02:12
0:02:12
 0:01:39
0:01:39
 0:20:15
0:20:15
 0:00:22
0:00:22
 0:00:16
0:00:16
 0:01:16
0:01:16
 0:12:47
0:12:47
 0:07:06
0:07:06
 0:01:06
0:01:06
 0:05:57
0:05:57
 0:04:07
0:04:07
 0:00:30
0:00:30
 0:03:29
0:03:29
 0:00:39
0:00:39
 0:16:36
0:16:36
 0:02:20
0:02:20