filmov
tv
Molality and Colligative Properties

Показать описание
Solute particles interfere with the physical processes a solution may undergo. These are known as the colligative processes of a solution. Ever wonder why we put salt on icy streets? Find out here!
#chemistry #maths #science #uziman #uzimancodes
Chapters:
0:00 - Molality and Colligative Properties
0:36 - What are Colligative Properties
1:29 - The outcome of adding solute
1:58 - What is Molality
3:31 - Examples of Molality
5:47 - Vapor Pressure Lowering
7:09 - Boling Point Elevation
8:11 - Freezing Point Depression
9:10 - K constants
11:58 - New Boiling and Freezing Points calculation
13:29 - Molality and Colligative Properties
#chemistry #maths #science #uziman #uzimancodes
Chapters:
0:00 - Molality and Colligative Properties
0:36 - What are Colligative Properties
1:29 - The outcome of adding solute
1:58 - What is Molality
3:31 - Examples of Molality
5:47 - Vapor Pressure Lowering
7:09 - Boling Point Elevation
8:11 - Freezing Point Depression
9:10 - K constants
11:58 - New Boiling and Freezing Points calculation
13:29 - Molality and Colligative Properties
Molality and Colligative Properties
Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure
Molarity, Molality, Volume & Mass Percent, Mole Fraction & Density - Solution Concentration ...
Molality and colligative properties
Molality and Colligative Properties
COLLIGATIVE PROPERTIES|Exhibition of Osmotic pressure, Relationship b/w Molality and Van hoff Factor
What's the Difference Between Molarity and Molality?
Solutions: Crash Course Chemistry #27
How to Calculate Molality of Solutions Examples, Practice Problems, Equation, Shortcut, Explanation
Molality | Solutions and Colligative properties | JEE | NEET | Chemistry - TG Campus
Colligative molality (mc)
Molality Problems - Solution and Colligative Properties - Chemistry Class 12
13.1 Introduction to Colligative Properties, the van't Hoff factor, and Molality
Molarity and Molality
Colligative Properties | Chemistry Matters
Colligative Properties Explained
13.3 Colligative Properties | General Chemistry
Colligative Properties | Physical Properties | Vapor Pressure | Osmotic Pressure | Molality
Molality #1
Molality concentration
Colligative Properties Worksheet B. Calculate the molality of a water solution if the freezing poin…...
Determining Molar Mass of Unknown using Freezing Point Depression (Colligative Properties)
Solutions - Colligative Properties
Calculate Moles of Solute from Molarity #science #chemistry #education #shorts #short
Комментарии
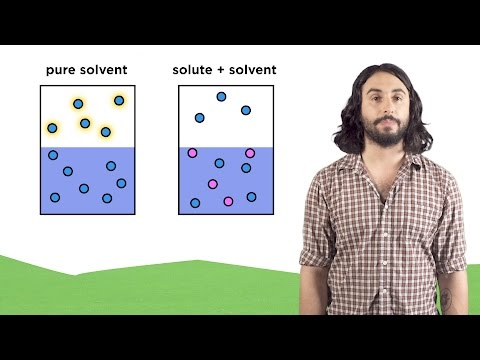 0:05:10
0:05:10
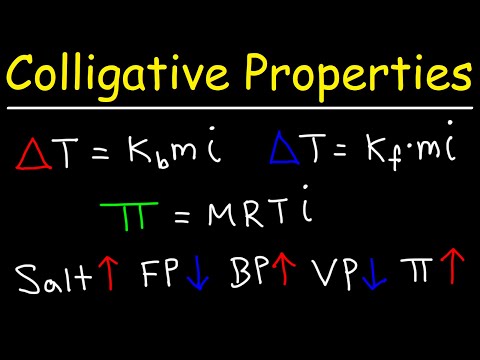 0:25:23
0:25:23
 0:31:25
0:31:25
 0:16:43
0:16:43
 0:14:06
0:14:06
 0:13:13
0:13:13
 0:05:11
0:05:11
 0:08:20
0:08:20
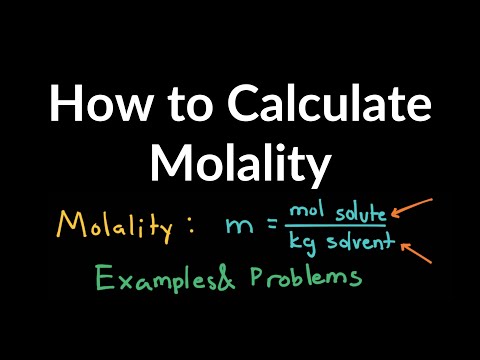 0:05:25
0:05:25
 0:08:12
0:08:12
 0:04:49
0:04:49
 0:03:32
0:03:32
 0:16:56
0:16:56
 0:00:16
0:00:16
 0:13:17
0:13:17
 0:17:44
0:17:44
 0:34:57
0:34:57
 0:21:10
0:21:10
 0:01:49
0:01:49
 0:08:05
0:08:05
 0:00:33
0:00:33
 0:04:41
0:04:41
 0:13:02
0:13:02
 0:00:38
0:00:38