filmov
tv
Bond Energy & Bond Length, Forces of Attraction & Repulsion - Chemistry
Показать описание
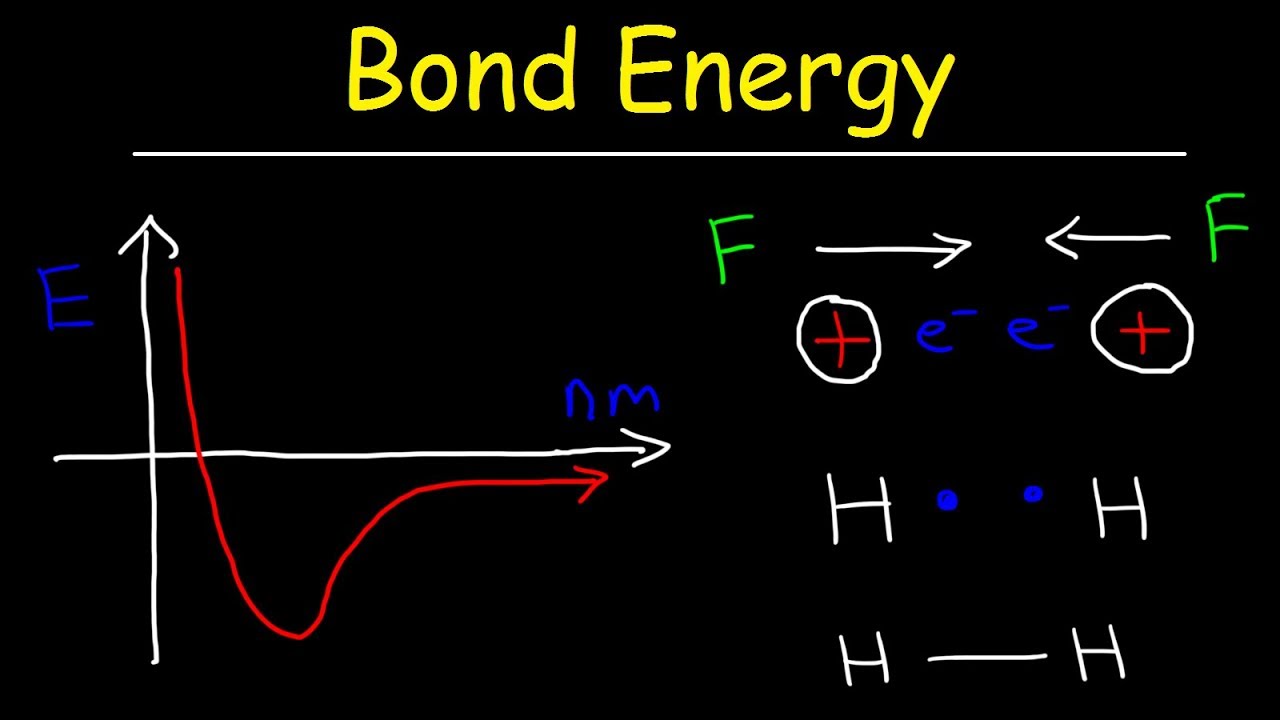
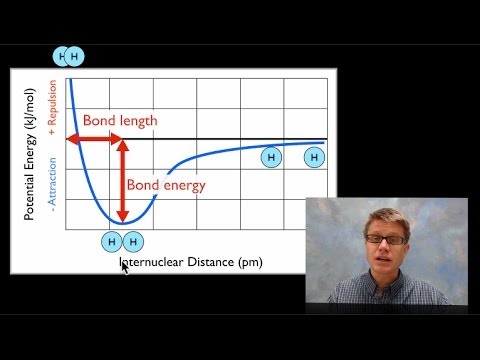
This video provides a basic introduction into bond energy and bond length. It explains how to determine the bond length of a molecule given a graph that shows the potential energy of two atoms and the internuclear distance in nm. It explains why atoms form bonds as they approach each other and why they repel each other when they get too close.
Ionization Energy:
Electron Affinity:
Atomic Radius:
Bond Energy & Bond Length:
Electronegativity:
Periodic Trends:
__________________________________
Polar & Nonpolar Covalent Bonding:
Bond Polarity & Dipole Moment:
Ionic Radius:
Lattice Energy:
Born Haber Cycle:
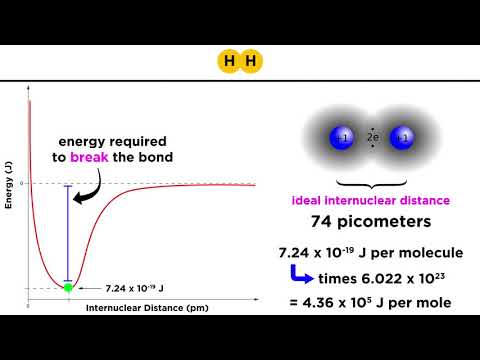
Bond Energy Calculations:
___________________________________
Lewis Structures - Mega Review:
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Ionization Energy:
Electron Affinity:
Atomic Radius:
Bond Energy & Bond Length:
Electronegativity:
Periodic Trends:
__________________________________
Polar & Nonpolar Covalent Bonding:
Bond Polarity & Dipole Moment:
Ionic Radius:
Lattice Energy:
Born Haber Cycle:
Bond Energy Calculations:
___________________________________
Lewis Structures - Mega Review:
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Комментарии
 0:06:42
0:06:42
 0:06:42
0:06:42
 0:11:36
0:11:36
 0:05:47
0:05:47
 0:14:49
0:14:49
 0:09:08
0:09:08
 0:13:45
0:13:45
 0:04:31
0:04:31
 0:46:50
0:46:50
 0:04:08
0:04:08
 0:04:17
0:04:17
 0:04:23
0:04:23
 0:28:51
0:28:51
 0:11:39
0:11:39
 0:10:18
0:10:18
 0:20:51
0:20:51
 0:51:15
0:51:15
 0:00:08
0:00:08
 0:12:37
0:12:37
 0:00:12
0:00:12
 0:18:25
0:18:25
 0:04:25
0:04:25
 0:12:01
0:12:01
 0:11:52
0:11:52