filmov
tv
Anne Bertolotti (MRC LMB) 3: A Platform to Identify Selective Protein Phosphatase Inhibitors
Показать описание
Kinases and phosphatases perform a balancing act in cells by adding and removing phosphate groups from proteins. Dr. Bertolotti shows us that inhibiting specific protein phosphatases can reduce misfolded protein accumulation and reduce neurodegenerative disease.
There are many processes and signals in cells that must be turned on and off, sometimes very quickly. How is this done? One important way is via post-translational modification of proteins such as phosphorylation or dephosphorylation. In her first talk, Dr. Anne Bertolotti introduces us to protein phosphatases, the enzymes that remove phosphate from proteins and work in opposition to protein kinases. She gives a brief history of the early experiments that showed that phosphatases are vital to regulating the stability, localization and interactions of many proteins. Bertolotti also describes more recent work demonstrating that protein phosphatases are split enzymes with a catalytic subunit and a subunit that determines substrate specificity. This selective subunit makes phosphatases exquisitely specific and attractive targets for drug development.
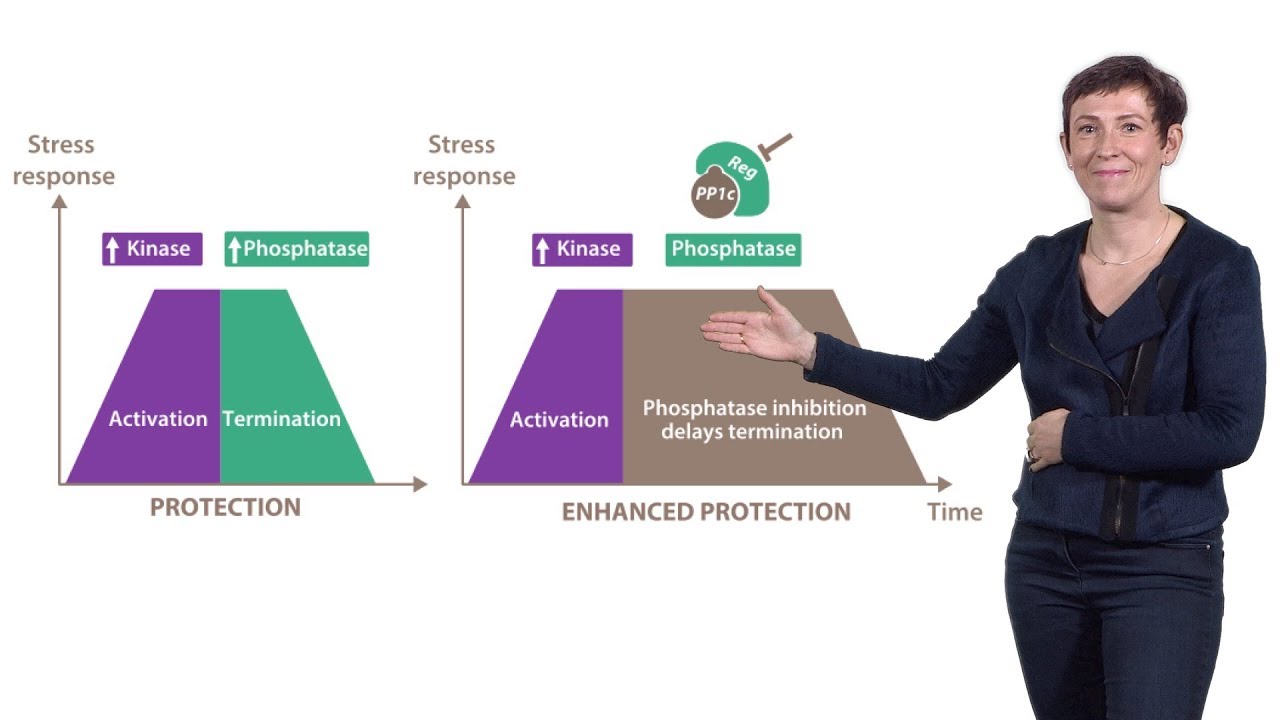
Bertolotti’s lab has had a long time interest in understanding protein folding and the role of misfolded proteins in neurodegenerative disease. In her second talk, Bertolotti explains how her lab found that selectively inhibiting the dephosphorylation of eIF2⍺, a translation initiation factor, led to a reduction in protein synthesis. Decreasing protein synthesis allowed cells to “catch up” with the degradation of misfolded proteins that may accumulate as a result of cell stress. Her lab went on to show that a selective small molecule phosphatase inhibitor had therapeutic effects in a mouse model of Charcot-Marie-Tooth disease; a disease that results from the accumulation of misfolded protein in the ER. This exciting result suggested that targeted inhibition of protein phosphatases may have therapeutic potential for neurodegenerative diseases.
In her third talk, Bertolotti describes a platform developed by her lab that has allowed them to rationally identify selective protein phosphatase inhibitors. Using this platform her lab identified a novel small molecule phosphatase inhibitor that blocks the accumulation of misfolded proteins in the cytosol or nucleus and showed the therapeutic effects of the molecule in a model of Huntington’s disease.
Speaker Biography:
Dr. Anne Bertolotti’s research focuses on understanding and preventing the deposition of misfolded proteins in cells, a hallmark of numerous neurological diseases. Bertolotti has been a group leader at the MRC Laboratory of Molecular Biology in Cambridge, UK since 2006. Prior to joining the LMB, she was an Associate Professor at Ecole Normale Superieure in Paris from 2001-2006. Bertolotti did her PhD training at the Institute of Genetics and Molecular and Cellular Biology (IGBMC) near Strasbourg, France and she was a post-doctoral fellow at the Skirball Institute of Biomolecular Medicine at NYU School of Medicine in New York.
Bertolotti was elected an EMBO Young Investigator in 2005 and an EMBO member in 2013. In 2014, she was awarded the Hooke Medal of the British Society for Cell Biology for her contributions to our understanding of abnormal protein folding. Bertolotti was elected a Fellow of the UK Academy of Medical Sciences in 2017.
Learn more about Bertolotti’s research here:
Комментарии
 0:34:15
0:34:15
 0:51:33
0:51:33
 0:17:06
0:17:06
 0:30:12
0:30:12
 0:19:19
0:19:19
 0:29:15
0:29:15
 0:04:54
0:04:54
 0:02:45
0:02:45
 0:03:58
0:03:58
 0:06:43
0:06:43
 0:06:14
0:06:14
 0:05:33
0:05:33
 0:01:05
0:01:05
 0:03:07
0:03:07
 0:03:13
0:03:13
 0:07:42
0:07:42
 0:04:48
0:04:48
 0:01:44
0:01:44
 0:00:07
0:00:07
 0:03:22
0:03:22
 0:02:41
0:02:41
 0:14:51
0:14:51
 0:00:22
0:00:22
 0:42:36
0:42:36