filmov
tv
Isothermal Expansion of Helium | Ideal Gas | Thermodynamics

Показать описание
Welcome to Engineering Hack! In today's video we are tackling a problem in which we have an isothermal transformation. An isothermal is a process in which the temperature does not change. This render some interesting results when we take into account an ideal gas (that is, a gas that follows the PV =mRT equation). This is an introductory thermodynamics problem still dealing with ideal gasses (noble gas helium). In ideal gas problems, the idea is generally using the ideal gas equation (PV = m R T) to find all properties of a given state. Then, move on to the next state and completely define it. This is no different. Have a go before watching the video. Let me know if you have any questions.
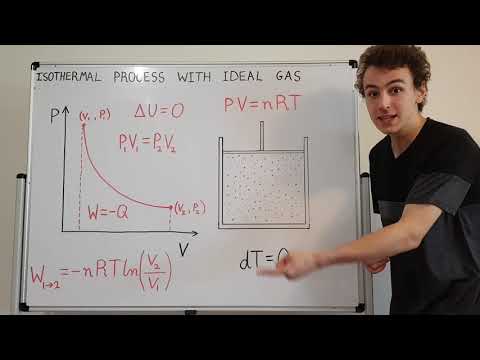
Problem statement: 3𝑘𝑔 of helium undergoes isothermal expansion. The helium has an initial pressure of 500𝑘𝑃𝑎 and an initial volume of 4𝑚³. The final volume of the helium is 5.2𝑚³.
a) Find the initial temperature of the helium in 𝐾.
b) Find the final pressure of the helium in 𝑘𝑃𝑎.
c) Find the work done by the system in 𝑘𝐽.
d) Draw the process on a 𝑃-𝑉 diagram with respect to the initial isotherm.
Take 𝑅helium =2.077 kJ/kg K.
Answer:
a) 321 K
b) 384.6 kPa
c) 524.8 kJ
d) Drawing at 07:30
To learn more please watch the video till end and if you have a question just ask in the comments section.
If you like the video, please SUBSCRIBE and don't forget to press the bell 🔔, 👍like, comment and share. ---------------------------
---------------------------
▶️Watch More Videos:
---------------------------
#thermodynamics #Engineeringuniversity #problemsolving #EngineeringHack #specificvolume #saturatedmixture #engineeringsolutions #engineeringaustralia #engineering #saturatedliquid #propertytables #steamtables #university #saturatedvapor #qualitygas #puresubstance
Problem statement: 3𝑘𝑔 of helium undergoes isothermal expansion. The helium has an initial pressure of 500𝑘𝑃𝑎 and an initial volume of 4𝑚³. The final volume of the helium is 5.2𝑚³.
a) Find the initial temperature of the helium in 𝐾.
b) Find the final pressure of the helium in 𝑘𝑃𝑎.
c) Find the work done by the system in 𝑘𝐽.
d) Draw the process on a 𝑃-𝑉 diagram with respect to the initial isotherm.
Take 𝑅helium =2.077 kJ/kg K.
Answer:
a) 321 K
b) 384.6 kPa
c) 524.8 kJ
d) Drawing at 07:30
To learn more please watch the video till end and if you have a question just ask in the comments section.
If you like the video, please SUBSCRIBE and don't forget to press the bell 🔔, 👍like, comment and share. ---------------------------
---------------------------
▶️Watch More Videos:
---------------------------
#thermodynamics #Engineeringuniversity #problemsolving #EngineeringHack #specificvolume #saturatedmixture #engineeringsolutions #engineeringaustralia #engineering #saturatedliquid #propertytables #steamtables #university #saturatedvapor #qualitygas #puresubstance
 0:08:09
0:08:09
 0:10:23
0:10:23
 0:03:39
0:03:39
 0:08:11
0:08:11
 0:08:17
0:08:17
 0:00:54
0:00:54
 0:08:45
0:08:45
 0:03:07
0:03:07
 0:06:15
0:06:15
 0:17:59
0:17:59
 0:00:36
0:00:36
 0:09:47
0:09:47
 0:00:52
0:00:52
 0:22:58
0:22:58
 0:02:43
0:02:43
 0:10:43
0:10:43
 0:01:51
0:01:51
 0:00:27
0:00:27
 0:05:38
0:05:38
 0:03:26
0:03:26
 0:00:48
0:00:48
 0:04:51
0:04:51
 0:03:02
0:03:02
 0:02:55
0:02:55