filmov
tv
Partial Miscibility and the Lever Rule L23 4449

Показать описание
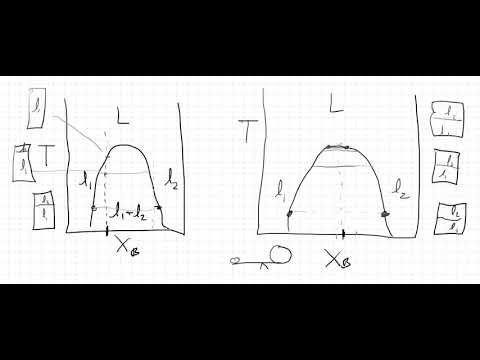
Partial miscibility and liquid phase separation is analyzed in detail. An extensive presentation of the lever rule for characterizing the layers is presented.
Bonus material. The thermodynamic cause of phase separation is explored using the Hansen Solubility Parameter theory. One can determine the asymmetry of the Enthalpy term lies in the difference in the molar volumes of the components of the mixture. These differing molar volumes indicate a substantial difference in intermolecular attractions in the pure substances. This leads to a temperature-dependent miscibility and a upper consolate temperature.
Background
These are videos of Dr. Williams’ CHEM 4449 Physical Chemistry Lectures at Sam Houston State University in the Spring of 2018. They are provided for your benefit for studying if you are a student or for your curiosity if you are not a student.
This course begins with the quantum world established in the first semester CHEM 4448 and explores the statistical basis of thermodynamics, classical thermodynamics, heat engines, phase diagrams, fluid properties, transport phenomena, and energy sources, sinks, and conversions on a global scale. It is a fascinating trip from the quantum to the universe in 3.5 months.
Keywords
SHSU, Quantum Mechanics, Statistical Thermodynamics, Heat, Work, Internal Energy, Entropy, Enthalpy, Gibbs Free Energy, Helmholtz Free Energy, Equilibrium, Heat Engines, Phase Diagrams, Azeotrope, Partially Miscible Liquids, Eutectic, Contact Angle, Hansen Solubility Parameters, Density, Surface Tension, Surface Energy, Viscosity, Vapor Pressure, Molar Volume, Carnot Cycle, State Variables, Coal, Crude Oil, Natural Gas, Nuclear Power
Bonus material. The thermodynamic cause of phase separation is explored using the Hansen Solubility Parameter theory. One can determine the asymmetry of the Enthalpy term lies in the difference in the molar volumes of the components of the mixture. These differing molar volumes indicate a substantial difference in intermolecular attractions in the pure substances. This leads to a temperature-dependent miscibility and a upper consolate temperature.
Background
These are videos of Dr. Williams’ CHEM 4449 Physical Chemistry Lectures at Sam Houston State University in the Spring of 2018. They are provided for your benefit for studying if you are a student or for your curiosity if you are not a student.
This course begins with the quantum world established in the first semester CHEM 4448 and explores the statistical basis of thermodynamics, classical thermodynamics, heat engines, phase diagrams, fluid properties, transport phenomena, and energy sources, sinks, and conversions on a global scale. It is a fascinating trip from the quantum to the universe in 3.5 months.
Keywords
SHSU, Quantum Mechanics, Statistical Thermodynamics, Heat, Work, Internal Energy, Entropy, Enthalpy, Gibbs Free Energy, Helmholtz Free Energy, Equilibrium, Heat Engines, Phase Diagrams, Azeotrope, Partially Miscible Liquids, Eutectic, Contact Angle, Hansen Solubility Parameters, Density, Surface Tension, Surface Energy, Viscosity, Vapor Pressure, Molar Volume, Carnot Cycle, State Variables, Coal, Crude Oil, Natural Gas, Nuclear Power
 0:44:20
0:44:20
 0:24:18
0:24:18
 0:22:29
0:22:29
 0:48:43
0:48:43
 0:43:03
0:43:03
 0:01:00
0:01:00
 0:26:25
0:26:25
 0:00:54
0:00:54
 0:23:25
0:23:25
 0:05:31
0:05:31
 0:03:08
0:03:08
 0:07:21
0:07:21
 0:11:51
0:11:51
 0:05:00
0:05:00
 0:22:44
0:22:44
 0:09:02
0:09:02
 0:18:35
0:18:35
 0:42:24
0:42:24
 0:13:46
0:13:46
 0:03:52
0:03:52
 0:15:41
0:15:41
 0:10:55
0:10:55
 0:13:54
0:13:54
 0:09:44
0:09:44