filmov
tv
Liquid-Liquid Extraction

Показать описание
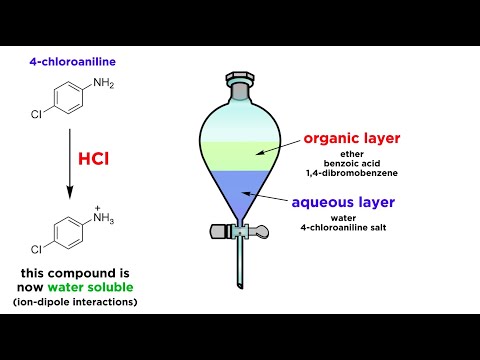
Separation techniques are important in chemistry, and they won't always be as easy as filtration. Sometimes we need to separate two compounds that are dissolved in the same solution. Often we can take advantage of a difference in solubility or reactivity to perform an extraction. This is where one component is pulled into another solvent that is immiscible with the first, and we can separate the two layers using a separatory funnel. There is a lot to learn to do this efficiently, so let's get a closer look at how to perform this technique!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Liquid-Liquid Extraction
Liquid Liquid Extraction
Liquid/Liquid Extraction
Liquid-Liquid Extraction
Separating Components of a Mixture by Extraction
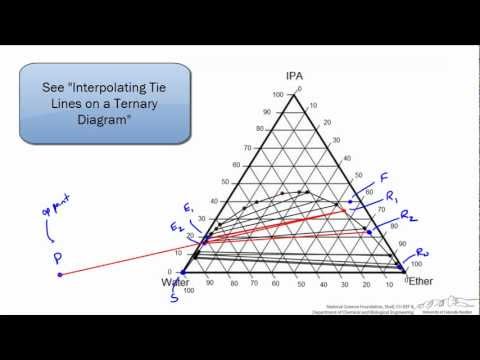
Hunter-Nash Method: Liquid-Liquid Extraction
A Short Liquid-Liquid Extraction Demonstration
What Exactly is Liquid-Liquid Extraction
The Transition: Leveraging Liquid Gold
Liquid-Liquid Extraction Material Balance
Liquid-liquid extraction
CHEM117 04 Liquid Liquid Extraction Fundamentals
Retrosynthesis and Liquid-Liquid Extraction: Crash Course Organic Chemistry #34
LIQUID-LIQUID EXTRACTION -UNDERSTANDING TERNARY DIAGRAM
Performing a Liquid-Liquid Extraction Using a Separatory Funnel
Performing Liquid-Liquid Extraction in a Test Tube
Introduction to Liquid-Liquid Extraction
Liquid-liquid extraction #extraction #liquid #separatingfunnel #separation #shorts
Lec 10: Introduction to liquid-liquid extraction, liquid-liquid equilibria
Liquid - Liquid Extraction
Liquid-Liquid Extraction
LIQUID-LIQUID EXTRACTION
A-Level Pre-Lab Video for Using a Separating Funnel
Liquid - Liquid Extraction | Mass Transfer by Arpit Gaur Sir | CHEMICAL ENGINEERING
Комментарии
 0:10:57
0:10:57
 0:02:53
0:02:53
 0:11:25
0:11:25
 0:02:38
0:02:38
 0:10:09
0:10:09
 0:09:02
0:09:02
 0:07:29
0:07:29
 0:18:16
0:18:16
 0:24:21
0:24:21
 0:07:11
0:07:11
 0:01:49
0:01:49
 0:26:46
0:26:46
 0:12:45
0:12:45
 0:18:35
0:18:35
 0:02:08
0:02:08
 0:01:20
0:01:20
 0:46:49
0:46:49
 0:00:43
0:00:43
 0:44:33
0:44:33
 0:07:48
0:07:48
 0:33:10
0:33:10
 0:10:09
0:10:09
 0:04:11
0:04:11
 0:14:35
0:14:35