filmov
tv
Radical Reactions & Hammond's Postulate: Crash Course Organic Chemistry #19

Показать описание
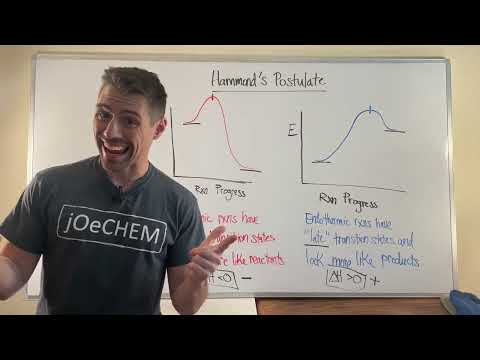
Throughout this series we’ve mostly talked about pairs of electrons, but electrons don’t always have a buddy. An atom or group of atoms with a single unpaired electron is called a radical. In this episode of Crash Course Organic Chemistry, we’ll learn all about radicals including the three key steps in a radical reaction and Hammond’s Postulate, an important tool to help us understand these reactions. We’ll also see ways radicals can react with alkanes, alkenes, and alkynes.
Episode Sources:
Davies, K. J., & Doroshow, J. H. (1986). Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. Journal of Biological Chemistry, 261(7), 3060-3067.
Ball, P. (Interview with Nick Lane) Yes, life in the fast lane kills you. May 5, 2016.
Nimse, S. B., & Pal, D. (2015). Free radicals, natural antioxidants, and their reaction mechanisms. Rsc Advances, 5(35), 27986-28006.
Santos-Sánchez, N. F., Salas-Coronado, R., Villanueva-Cañongo, C., & Hernández-Carlos, B. (2019). Antioxidant compounds and their antioxidant mechanism. In Antioxidants. IntechOpen.
Review of Vitamin C
Series Sources:
Brown, W. H., Iverson, B. L., Ansyln, E. V., Foote, C., Organic Chemistry; 8th ed.; Cengage Learning, Boston, 2018.
Bruice, P. Y., Organic Chemistry, 7th ed.; Pearson Education, Inc., United States, 2014.
Clayden, J., Greeves, N., Warren., S., Organic Chemistry, 2nd ed.; Oxford University Press, New York, 2012.
Jones Jr., M.; Fleming, S. A., Organic Chemistry, 5th ed.; W. W. Norton & Company, New York, 2014.
Klein., D., Organic Chemistry; 1st ed.; John Wiley & Sons, United States, 2012.
Louden M., Organic Chemistry; 5th ed.; Roberts and Company Publishers, Colorado, 2009.
McMurry, J., Organic Chemistry, 9th ed.; Cengage Learning, Boston, 2016.
Smith, J. G., Organic chemistry; 6th ed.; McGraw-Hill Education, New York, 2020.
Wade., L. G., Organic Chemistry; 8th ed.; Pearson Education, Inc., United States, 2013.
***
Watch our videos and review your learning with the Crash Course App!
Thanks to the following patrons for their generous monthly contributions that help keep Crash Course free for everyone forever:
Eric Prestemon, Mark, DAVID MORTON HUDSON, Perry Joyce, Isaac Liu, Scott Harrison, Mark & Susan Billian, Junrong Eric Zhu, Alan Bridgeman, Jennifer Smith, Matt Curls, Tim Kwist, Jonathan Zbikowski, Jennifer Killen, Sarah & Nathan Catchings, Brandon Westmoreland, team dorsey, Trevin Beattie, Eric Koslow, Indika Siriwardena, Khaled El Shalakany, Shawn Arnold, Siobhán, Ken Penttinen, Nathan Taylor, William McGraw, Jirat, Brian Thomas Gossett, Ian Dundore, Jason A Saslow, Jessica Wode, Caleb Weeks
__
Want to find Crash Course elsewhere on the internet?
Episode Sources:
Davies, K. J., & Doroshow, J. H. (1986). Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. Journal of Biological Chemistry, 261(7), 3060-3067.
Ball, P. (Interview with Nick Lane) Yes, life in the fast lane kills you. May 5, 2016.
Nimse, S. B., & Pal, D. (2015). Free radicals, natural antioxidants, and their reaction mechanisms. Rsc Advances, 5(35), 27986-28006.
Santos-Sánchez, N. F., Salas-Coronado, R., Villanueva-Cañongo, C., & Hernández-Carlos, B. (2019). Antioxidant compounds and their antioxidant mechanism. In Antioxidants. IntechOpen.
Review of Vitamin C
Series Sources:
Brown, W. H., Iverson, B. L., Ansyln, E. V., Foote, C., Organic Chemistry; 8th ed.; Cengage Learning, Boston, 2018.
Bruice, P. Y., Organic Chemistry, 7th ed.; Pearson Education, Inc., United States, 2014.
Clayden, J., Greeves, N., Warren., S., Organic Chemistry, 2nd ed.; Oxford University Press, New York, 2012.
Jones Jr., M.; Fleming, S. A., Organic Chemistry, 5th ed.; W. W. Norton & Company, New York, 2014.
Klein., D., Organic Chemistry; 1st ed.; John Wiley & Sons, United States, 2012.
Louden M., Organic Chemistry; 5th ed.; Roberts and Company Publishers, Colorado, 2009.
McMurry, J., Organic Chemistry, 9th ed.; Cengage Learning, Boston, 2016.
Smith, J. G., Organic chemistry; 6th ed.; McGraw-Hill Education, New York, 2020.
Wade., L. G., Organic Chemistry; 8th ed.; Pearson Education, Inc., United States, 2013.
***
Watch our videos and review your learning with the Crash Course App!
Thanks to the following patrons for their generous monthly contributions that help keep Crash Course free for everyone forever:
Eric Prestemon, Mark, DAVID MORTON HUDSON, Perry Joyce, Isaac Liu, Scott Harrison, Mark & Susan Billian, Junrong Eric Zhu, Alan Bridgeman, Jennifer Smith, Matt Curls, Tim Kwist, Jonathan Zbikowski, Jennifer Killen, Sarah & Nathan Catchings, Brandon Westmoreland, team dorsey, Trevin Beattie, Eric Koslow, Indika Siriwardena, Khaled El Shalakany, Shawn Arnold, Siobhán, Ken Penttinen, Nathan Taylor, William McGraw, Jirat, Brian Thomas Gossett, Ian Dundore, Jason A Saslow, Jessica Wode, Caleb Weeks
__
Want to find Crash Course elsewhere on the internet?
Комментарии
 0:12:01
0:12:01
 0:15:09
0:15:09
 0:03:04
0:03:04
 0:09:40
0:09:40
 0:07:45
0:07:45
 0:07:18
0:07:18
 0:09:00
0:09:00
 0:08:44
0:08:44
 0:02:50
0:02:50
 0:05:51
0:05:51
 0:06:41
0:06:41
 0:35:05
0:35:05
 0:05:45
0:05:45
 0:01:31
0:01:31
 0:15:45
0:15:45
 0:03:47
0:03:47
 0:35:57
0:35:57
 0:05:34
0:05:34
 1:25:48
1:25:48
 0:13:29
0:13:29
 0:14:08
0:14:08
 0:14:25
0:14:25
 0:01:02
0:01:02
 0:06:29
0:06:29