filmov
tv
When electromagnetic radiation of wavelength 300nm falls on the surface of sodium, electrons are....

Показать описание
NCERT Problem Page No. 43 Structure of Atom
Problem 2.8:- When electromagnetic radiation of wavelength 300nm falls on the surface of sodium, electrons are emitted with a kinetic energy of 1.68 X 105 J mol-1. What is the minimum energy needed to remove an electron from sodium? What is the maximum wavelength that will cause a photoelectron to be emitted?
Problem 2.8:- When electromagnetic radiation of wavelength 300nm falls on the surface of sodium, electrons are emitted with a kinetic energy of 1.68 X 105 J mol-1. What is the minimum energy needed to remove an electron from sodium? What is the maximum wavelength that will cause a photoelectron to be emitted?
When electromagnetic radiation of wavelength 300nm falls on the surface of sodium, electrons are....
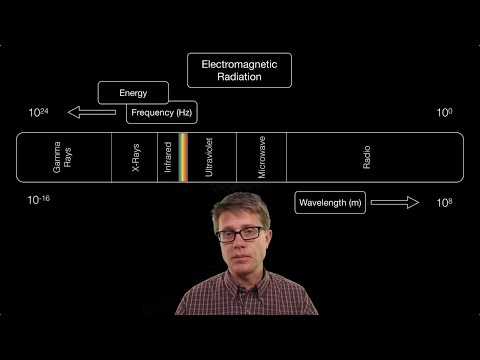
Electromagnetic Spectrum - Basic Introduction
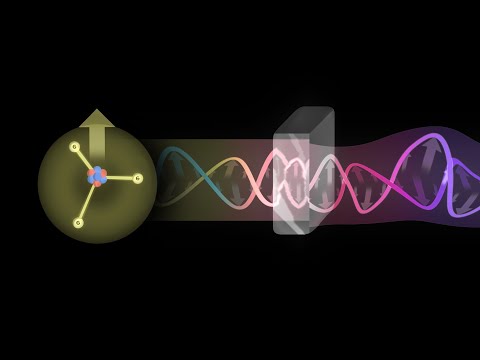
What is Light? Maxwell and the Electromagnetic Spectrum
Electromagnetic Radiation
Understanding Electromagnetic Radiation! | ICT #5
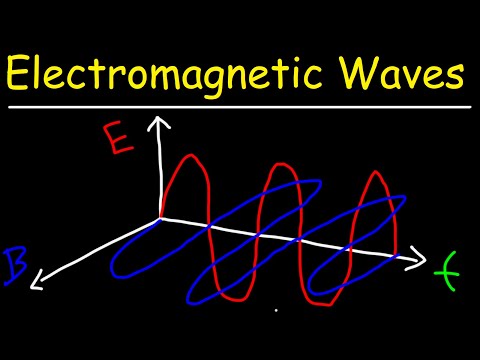
Electromagnetic Waves
The origin of Electromagnetic waves, and why they behave as they do
Electromagnetic radiation of wavelength 242 nm is just sufficient to ionize the sodium atom.
'Origins of Gravity, Electromagnetism and the Inverse Square Law'
When electromagnetic radiation of wavelength math xmlns=http://www.w3.org/1998/Math/MathMLmn300/...
The Electromagnetic Spectrum Introduction | Study Chemistry With Us
When electromagnetic radiation of wavelength 300nm falls on the surface of sodium, electrons are....
The Electromagnetic Spectrum | Visible Lights, Radio Waves and X-rays
Light waves, visible and invisible
Electromagnetic Radiation and Electromagnetic Spectrum | X-ray physics | Radiology Physics Course #7
GCSE Physics - Electromagnetic Waves #64
When electromagnetic radiation of wavelength 300 nm falls on the surface of sodium
Calculating the wavelength of light #chemistry #homework #science #shorts #sciencefacts
When electromagnetic radiation of wavelength \(300 nm\) falls on the surface of a metal, electro....
An easy way to remember the Electromagnetic Spectrum.
How to remember Electromagnetic Spectrum
When electromagnetic radiaiton of wavelength `300 nm` falls on the surface of sodium electrons a...
Energy from Wavelength: Electromagnetic Radiation Calculation
ALEKS: Interconverting the wavelength and frequency of electromagnetic radiation
Комментарии
 0:09:56
0:09:56
 0:03:56
0:03:56
 0:03:02
0:03:02
 0:07:29
0:07:29
 0:06:30
0:06:30
 0:12:05
0:12:05
 0:09:24
0:09:24
 1:29:31
1:29:31
 0:09:07
0:09:07
 0:11:37
0:11:37
 0:14:57
0:14:57
 0:00:57
0:00:57
 0:05:58
0:05:58
 0:08:58
0:08:58
 0:04:52
0:04:52
 0:16:06
0:16:06
 0:00:55
0:00:55
 0:06:16
0:06:16
 0:00:12
0:00:12
 0:00:17
0:00:17
 0:04:53
0:04:53
 0:04:43
0:04:43
 0:04:42
0:04:42