filmov
tv
Tim Hickling: Applying in vitro immunogenicity assays to predict clinical immunogenicity
Показать описание
Mastering Immunity Europe 2017
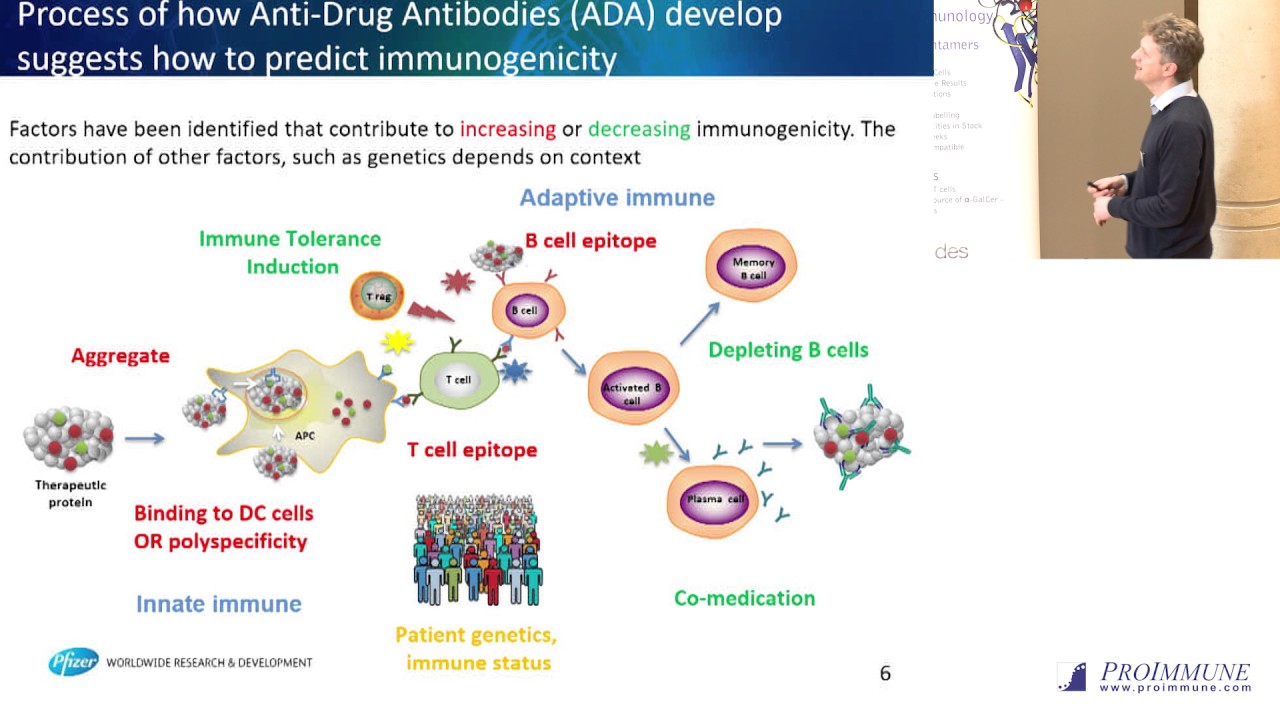
An immunogenicity risk assessment incorporates risk factors related to patients, products and the way the product is given to the patient. Prediction of the clinical impact of immunogenicity could be possible through a quantitative understanding of these risk factors and how the immune system operates. In vitro assays contribute to this quantitative understanding of risk and can be used during the discovery phase to inform risk mitigation strategies such as selection of lower risk leads.
Tim Hickling leads the Immunogenicity Sciences group in Pfizer that is responsible for immunogenicity risk assessments and developing predictive methods for immune responses. Tim joined Pfizer in the UK in 2007 to develop Vaccines before switching to unwanted immunogenicity, and the USA, in 2011. He had previously obtained his Biochemistry degree and Immunology Doctorate from the University of Oxford, U.K. and was a Lecturer in Virology at the University of Nottingham, U.K. Tim is an associate editor for the AAPS Open journal and is a co-leader for the IMI ABIRISK consortium.
An immunogenicity risk assessment incorporates risk factors related to patients, products and the way the product is given to the patient. Prediction of the clinical impact of immunogenicity could be possible through a quantitative understanding of these risk factors and how the immune system operates. In vitro assays contribute to this quantitative understanding of risk and can be used during the discovery phase to inform risk mitigation strategies such as selection of lower risk leads.
Tim Hickling leads the Immunogenicity Sciences group in Pfizer that is responsible for immunogenicity risk assessments and developing predictive methods for immune responses. Tim joined Pfizer in the UK in 2007 to develop Vaccines before switching to unwanted immunogenicity, and the USA, in 2011. He had previously obtained his Biochemistry degree and Immunology Doctorate from the University of Oxford, U.K. and was a Lecturer in Virology at the University of Nottingham, U.K. Tim is an associate editor for the AAPS Open journal and is a co-leader for the IMI ABIRISK consortium.
 0:35:21
0:35:21
 0:33:11
0:33:11
 0:36:50
0:36:50
 0:52:05
0:52:05
 0:49:32
0:49:32
 0:11:27
0:11:27
 0:31:06
0:31:06
 0:03:38
0:03:38
 0:31:11
0:31:11
 0:44:22
0:44:22
 0:15:01
0:15:01
 0:17:30
0:17:30
 0:13:02
0:13:02
 0:21:27
0:21:27
 0:30:59
0:30:59
 0:08:52
0:08:52
 0:05:10
0:05:10
 0:28:56
0:28:56
 0:20:21
0:20:21
 0:17:03
0:17:03
 0:22:34
0:22:34
 0:37:51
0:37:51
 0:33:27
0:33:27
 0:14:58
0:14:58