filmov
tv
Activation Energy

Показать описание
The red phosphorus on the end of matches need activation energy to react with the oxygen in the air. The heat energy from the hot plate provide enough energy to activate the chemical reaction.
Activation Energy
Activation energy: Kickstarting chemical reactions - Vance Kite
What IS activation energy, really?
Activation Energy
Enzymes and activation energy | Biomolecules | MCAT | Khan Academy
R2.2.4 Activation energy
Function of Enzymes: Substrate, Active Site & Activation Energy
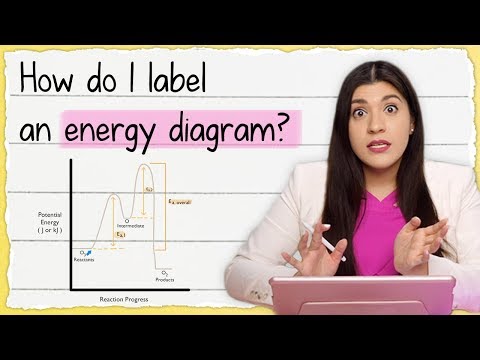
Energy Diagrams, Catalysts, and Reaction Mechanisms
The Return of the Ancient Lover 2 @MultiverseRecords_#moon #frequency #attunement #activation
Collision Theory - Arrhenius Equation & Activation Energy - Chemical Kinetics
Activation Energy and Catalysts
Catalysts and Activation Energy
Activation Energy (and exploding bags of Chlorine) - Periodic Table of Videos
Activation Energy Explained By Tariq Pathan
Enzymes' Effect on Activation Energy and Free Energy
Define Activation energy| World of Chemistry
GCSE Chemistry - Exothermic and Endothermic Reactions #43
Energy and Chemical Change grade 11: Activation energy, heat of reaction, catalyst and MORE!
Activation Energy
The Power of Activation Energy - Mel Robbins
what is Activation Energy ??? / Definition / Significance / How enzymes Lower activation energy
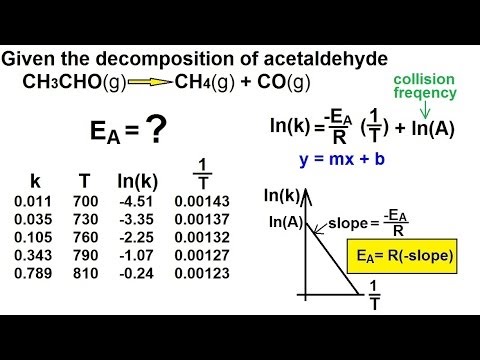
Chemistry - Chemical Kinetics (25 of 30) Determining the Activation Energy
How to Calculate Activation Energy (Ea) with Arrhenius Equation
Exothermic Energy Diagram: Activation Energy, Transition States and Enthalpy Change - TUTOR HOTLINE
Комментарии
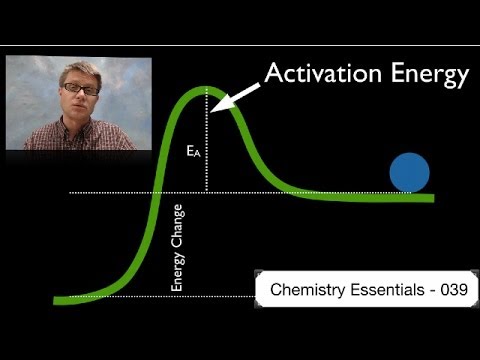 0:04:52
0:04:52
 0:03:23
0:03:23
 0:23:34
0:23:34
 0:05:32
0:05:32
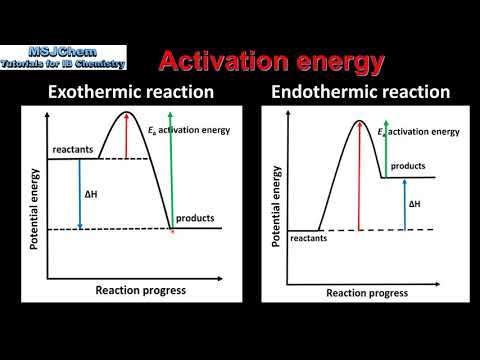 0:03:02
0:03:02
 0:02:41
0:02:41
 0:05:23
0:05:23
 0:00:38
0:00:38
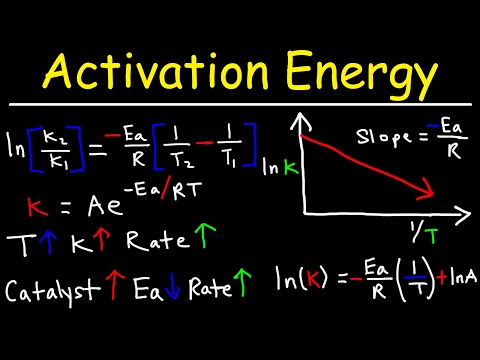 0:31:50
0:31:50
 0:03:36
0:03:36
 0:03:44
0:03:44
 0:10:26
0:10:26
 0:04:39
0:04:39
 0:12:14
0:12:14
 0:01:50
0:01:50
 0:05:21
0:05:21
 0:10:41
0:10:41
 0:07:04
0:07:04
 0:01:00
0:01:00
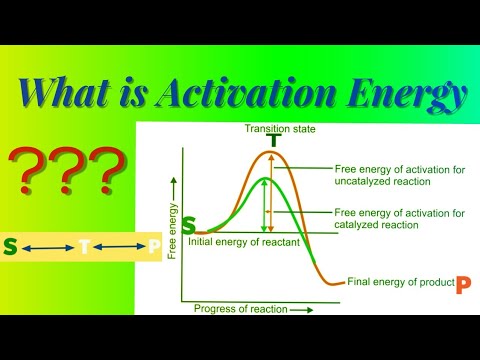 0:03:00
0:03:00
 0:05:59
0:05:59
 0:15:23
0:15:23
 0:08:40
0:08:40