filmov
tv
Class XII Chemistry Practical Simulation. FOR FULL VIDEO CHECK MY INSTAGRAM HANDLE @khuranasanket9

Показать описание
Class XII Chemistry Practical Simulation. FOR FULL VIDEO CHECK MY INSTAGRAM HANDLE @khuranasanket9
Colorful chemistry magic
Simulation with technology in Chemistry
Setting up and Performing a Titration
Boyle’s Law
Learn Chemistry Practicals Using SimuLab
Introduction to lab - lab simulation
Virtual Simulation for Molarity for Chemistry Class | Science Experiment
Voltaic cell | How does it work?
Total Internal Reflection || Experiments || Infinite Engineers
Short414 Simulation Under Microscope
3 Websites for Science Simulations for Teachers
Saponification: The process of Making Soap - MeitY OLabs
Segment of a Nanoreactor simulation
WSA-m 2015: iChemist - Titration Simulator
Chemical reactions between metals and water
Galvanic cells explained -in UNDER 5 MINUTES.
Galvanic Cell.swf
UPSC VS IIT JEE 🥵 #iitstatus #motivation #toppers #iitjee #jeemains #upscstatus #neet #nit #jee
Real simulation of a chain reaction in a radioactive substance #science #chemistry #chemisphere
Teaching chemistry with a simulation program 👏 #education #tech #innovation #shorts #techaminute
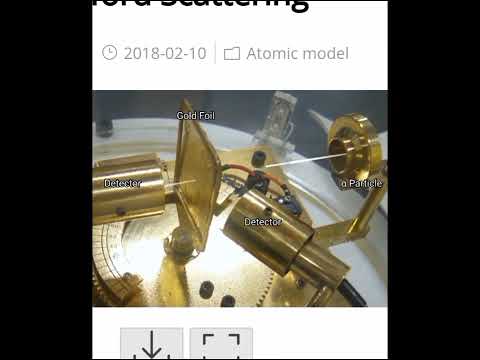
Rutherford Scattering Experiment | Alpha Scattering Simulation | Atomic Structure #imagineyourstudy
Paramagnetism and Diamagnetism
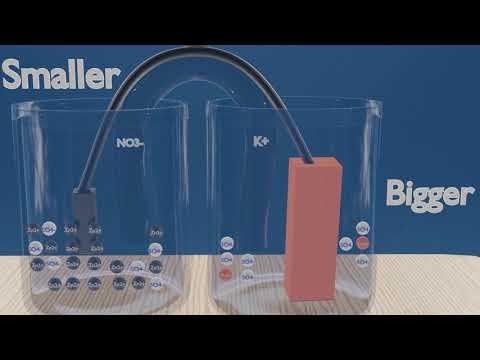
Electrochemical cell: The Daniell cell
Комментарии
 0:01:01
0:01:01
 0:00:30
0:00:30
 0:00:16
0:00:16
 0:06:53
0:06:53
 0:00:15
0:00:15
 0:01:40
0:01:40
 0:04:04
0:04:04
 0:04:29
0:04:29
 0:04:10
0:04:10
 0:00:29
0:00:29
 0:00:12
0:00:12
 0:09:30
0:09:30
 0:03:08
0:03:08
 0:00:17
0:00:17
 0:00:37
0:00:37
 0:01:01
0:01:01
 0:03:41
0:03:41
 0:02:08
0:02:08
 0:00:14
0:00:14
 0:00:36
0:00:36
 0:00:22
0:00:22
 0:00:25
0:00:25
 0:04:31
0:04:31
 0:02:22
0:02:22