filmov
tv
The van der Waals equation | Khan Academy
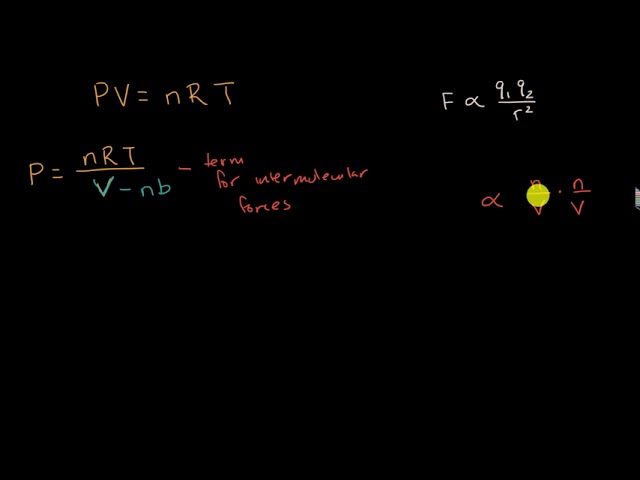
Показать описание
Keep going! Check out the next lesson and practice what you’re learning:
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
The van der Waals equation | Khan Academy
Non-Ideal Gases and the Van der Waals Equation
The van der Waals equation explained
Real gases and the van der Waals equation | Physical Processes | MCAT | Khan Academy
The van der Waals Equation | Physical Chemistry I | 014
van der Waals example
10.4 Real Gases & the Van der Waals Equation | General Chemistry
Van Der Waals Equation
Van Der Waals Equation | Real Gas Equation | Volume Correction | Pressure Correction | Sir Irfan
van der Waals Critical Point
van der Waals Equation for Non-Ideal Gases
Van Der Waals Forces
Real Gases and van der Waals Equation
Implicit Differentiation |Van der Waals Equation| dV/dP, dP/dT, dV/dT |Challenging question|Calculus
Not Ideal: The Van der Waals equation of state (Part 2)
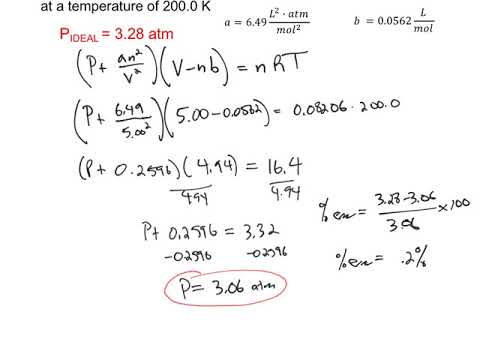
Finding the Van Der Waals Constants.
Van der Waals Equation of State
Van der Waals equation for real gases | Equation of state | Real gas | Lecture 11
Chapter 16: Using the van der Waals Equation | CHM 307 | 023
Critical Constants: Van der Waals Equation | Physical Chemistry I | 018
Van der Waals equation of state #VanDerWaalsEquationOfState #IdealGasLaw #Thermodynamics
Ch#4 | Lec#10 | Vander waal Equation | Real gas Equation | #Chemistry 11
Thermodynamics - Van der waals equation
Van der Waal's Equation | Derivation with Volume and Pressure Correction #VanderwaalEquation
Комментарии
 0:05:17
0:05:17
 0:08:09
0:08:09
 0:03:32
0:03:32
 0:07:17
0:07:17
 0:16:28
0:16:28
 0:08:09
0:08:09
 0:12:19
0:12:19
 0:01:48
0:01:48
 0:01:00
0:01:00
 0:11:08
0:11:08
 0:04:44
0:04:44
 0:10:38
0:10:38
 0:08:15
0:08:15
 0:19:10
0:19:10
 0:20:50
0:20:50
 0:12:31
0:12:31
 0:03:55
0:03:55
 0:12:24
0:12:24
 0:05:57
0:05:57
 0:13:14
0:13:14
 0:00:12
0:00:12
 0:21:37
0:21:37
 0:01:17
0:01:17
 0:19:57
0:19:57