filmov
tv
Method Validation, Fitness for purpose of analytical methods Part-1

Показать описание
What is Analytical method validation?
What is different between Method Validation and verification?
Why Method Validation necessary?
When should methods be validated or verified?
How should method be validate?
What is different between Method Validation and verification?
Why Method Validation necessary?
When should methods be validated or verified?
How should method be validate?
Method Validation, Fitness for purpose of analytical methods Part-1
Method Validation Fitness for purpose of analytical methods Part 2
Selectivity, Specificity Analytical Method validation Fitness for purpose of Analytical method Part3
Planning method validation studies
If Your Method Fit for Purpose - A Dive into Validation
Analytical Method Validation
Medical Device Test Method Validation Full Medical Device (Online Course with certification)
Analytical Method Validation software SMARTENOVAL ICH Q2 (R2) | Bruno Boulanger
ASQ Inspection Division Conference 2017 Dr Wayne Taylor Test Method Validation
Validation of qualitative methods | Cut off limit | sensitivity rate | Unreliability region
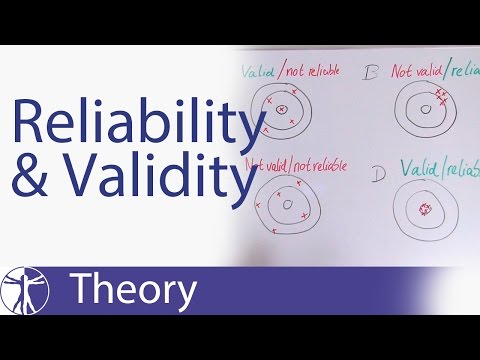
Reliability & Validity Explained
Method validation - overview of accreditation requirements - Lorens Sibbesen
Test Method Validation Standards and Guidelines | Medical Devices
Analytical Method Validation # ICH Guidlines
Analytical Method Development & Validation
2.A : Precision Vs. Accuracy
Method Validation Parameters in describes
Method validation | Lecture 2
3-Difference between method validation and verification
1- Introduction to method validation
Analytical Method Validation | Oops ! || 🇮🇳
Validation Parameters of Analytical Methods as per ICH guidelines: PART-1
Great Example of Hypergamy
Specificity,Analytical method Validation//Specificity use in AMV...
Комментарии
 0:06:46
0:06:46
 0:08:16
0:08:16
 0:12:19
0:12:19
 0:26:48
0:26:48
 0:34:44
0:34:44
 0:05:49
0:05:49
 0:01:18
0:01:18
 0:08:52
0:08:52
 0:48:20
0:48:20
 0:21:51
0:21:51
 0:02:57
0:02:57
 0:25:53
0:25:53
 0:00:55
0:00:55
 0:46:32
0:46:32
 0:02:17
0:02:17
 0:35:41
0:35:41
 0:18:31
0:18:31
 0:13:06
0:13:06
 0:12:10
0:12:10
 0:02:32
0:02:32
 0:00:15
0:00:15
 0:36:25
0:36:25
 0:00:54
0:00:54
 0:00:15
0:00:15