filmov
tv
Atomic packing factor in body centred cubic

Показать описание
In this video, Parisa works through the calculation of the atomic packing factor (APF) for the body centred cubic (BCC) crystal structure.
Atomic packing factor in body centred cubic
Atomic packing Factor of FCC and BCC Unit cell with animation
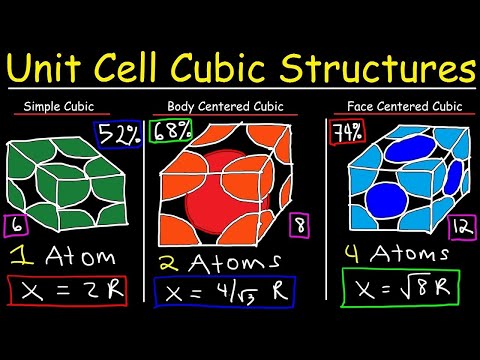
Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu
Atomic Packing Factor (A.P.F) OF S.C , B.C.C , F.C.C & CO-ORDINATION NUMBERS
BCC crystal structure atomic packing factor
APF of BCC
Atomic Packing Factor of BCC
Atomic packing factor
Atomic Packing Factor (crystallography )
Atomic Packing Factor For S.C, B.C.C And F.C.C In A Unit Cell
Atomic Packing Factor
Atomic Packing Factor for BCC: How to Find the Atomic Packing Factor (APF) of a BCC #jonahemmanuel
Atomic Packing Factor-Body Centered Cube(BCC)
Atomic packing factor for Body centered cubic (BCC)
Packing fraction of SCC, FCC and BCC cubic unit cells- Solid state chemistry
Atomic Packing Factor
Atomic packing factor in BCC
Atomic Packing Factor | Engineering Materials
Atomic packing factor of FCC
SCC BCC & FCC Unit Cell ||Explain SCC BCC and FCC Unit Cell ||what is scc unit cell ||Bcc unit c...
Hexagonal Close Packed Crystal Structure
Calculation of Packing Fraction of cubic Unit Cells | Solids | Chemistry | Khan Academy
Crystal Structures Simple Cubic Structure
Crystal Structures BCC Body Centered Cubic #shorts #metallurgy #materialscience
Комментарии
 0:02:18
0:02:18
 0:05:26
0:05:26
 0:17:22
0:17:22
 0:29:16
0:29:16
 0:14:08
0:14:08
 0:03:32
0:03:32
 0:20:00
0:20:00
 0:20:45
0:20:45
 0:03:01
0:03:01
 0:01:41
0:01:41
 0:51:53
0:51:53
 0:12:22
0:12:22
 0:05:27
0:05:27
 0:03:27
0:03:27
 0:18:30
0:18:30
 0:21:19
0:21:19
 0:02:08
0:02:08
 0:02:14
0:02:14
 0:11:17
0:11:17
 0:00:16
0:00:16
 0:04:02
0:04:02
 0:13:50
0:13:50
 0:00:16
0:00:16
 0:00:16
0:00:16