filmov
tv
Resonance & Bond Order

Показать описание
Made with Explain Everything
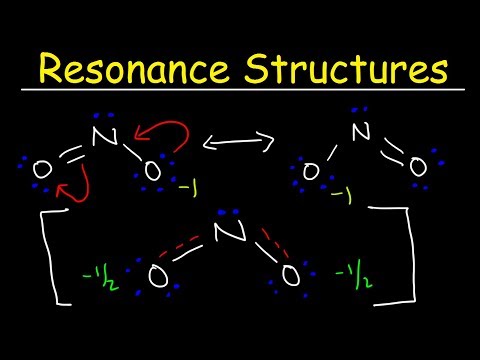
Bond Order and Resonance Structures
Resonance & Bond Order
Bond Order in Resonance Structures (VSEPR) | CHEM101
Resonance and Bond Order
Resonance Structures, Basic Introduction - How To Draw The Resonance Hybrid, Chemistry
RESONANCE Of Polyatomic Ions & Bond Order
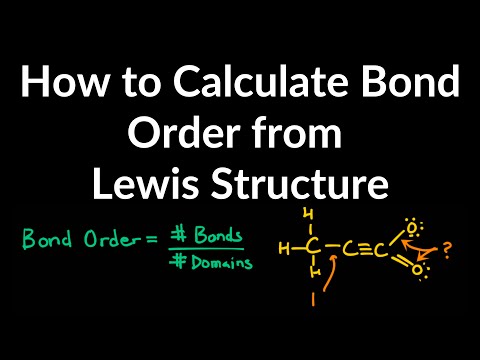
How to Calculate Bond Order From Lewis Structures Examples, Practice Problems, Explained, Shortcut
Calculation of Bond Order in Resonating Species.
Resonance and bond order
Resonance and Bond Order
Lewis Diagrams, Resonance Structures, Bond Order - AP Chemistry
Bond Order|Resonating Structures|3.5 Minutes
Structure, Bond Order, Formal Charge, Oxidation State & Resonance
Resonance and Bond Order. Echem L11 S04
3.4 - Resonance, Formal Charge, & Bond Order
3.4 - Resonance, Formal Charge, Bond Order
Bond order and resonance structures
Lewis Structures, Formal Charge, Resonance, Bond Order/Bond Length
12.2hb - Resonance and Bond Order
Resonance & Bond Order
bond order of lithium #shzclasses#viralshorts#sortsvideo#bondorder#chemistry
Resonance Structures & Bond Order Calculations, basics of organic chemistry
Bond order ||fractional bond order || resonance structure || chemical bonding ||Organic chemistry
chemical bonding class 11 | Resonance concept | Bond order
Комментарии
 0:05:25
0:05:25
 0:07:36
0:07:36
 0:09:01
0:09:01
 0:05:34
0:05:34
 0:10:31
0:10:31
 0:29:46
0:29:46
 0:05:10
0:05:10
 0:10:43
0:10:43
 0:13:23
0:13:23
 0:13:59
0:13:59
 0:02:09
0:02:09
 0:03:39
0:03:39
 0:15:47
0:15:47
 0:03:08
0:03:08
 0:07:31
0:07:31
 0:07:31
0:07:31
 0:06:01
0:06:01
 0:14:15
0:14:15
 0:07:07
0:07:07
 0:16:47
0:16:47
 0:00:57
0:00:57
 0:09:02
0:09:02
 0:08:55
0:08:55
 0:15:20
0:15:20