filmov
tv
Pharmacy Calculations for Technicians - Concentrations and Dilutions

Показать описание
This video explains a couple of methods to solve concentration and dilution problems in the pharmacy.
Pharmacy Calculations for Technicians - Dosage Calculations
Pharmacy Calculations: Days Supply - PTCB PTCE, NAPLEX, NCLEX Test Prep CPhT Pharmacy Technician
Pharmacy Calculations for Technicians - Percents, Percent Strength, Ratio Strength
Math Calculations for Pharmacy Technician Exams Made Easy! [PTCB, PTCE prep, PEBC Tech MCQ],
Drug Calculations Made Ridiculously Easy
Pharmacy Calculations for Technicians - Unit Conversions
Pharmacy Calculations for Technicians - Introduction - Please View First
Pharmacy Calculations: Conversions - PTCB PTCE CPhT Pharmacy Tech NAPLEX NCLEX Nursing Test Prep
Pharmacy Calculations for Technicians - IV Drip Rates
Pharmacy Math (1/2)
Pharmacy Calculations for Technicians-The Book
Pharmacy Calculations for Technicians - Concentrations and Dilutions
Pharmacy calculations for Technicians - Milliequivalents
Pharmacy Calculations: Concentrations - PTCB PTCE, NAPLEX, NCLEX Test Prep CPhT Pharmacy Technician
Pharmacy Calculations: Dilutions - Pharmacy Math PTCB PTCE NAPLEX NCLEX Test Prep CPhT Technician
Pharmacy Calculations: Ratios and Proportions - PTCB PTCE NAPLEX NCLEX Test Prep CPhT Pharmacy Tech
Pharmacy Calculations: IV Flow Rates - PTCB CPhT PTCE Math Pharmacy Technician NAPLEX Test Prep
Pharmacy Calculations for Technicians - Percentage of Error
Math Calculations for Pharmacy Technicians: Full Video Presentation Series (10 Part Lecture)
COMMENT THE RIGHT ANSWER #pharmacy #pharmacist #Pharmacology #calculation #hospital #clinical
IV Drug Calculation
Pharmacy Calculations for Technicians - Powder Volume Problems
Pharmacy Technician Basic Math Skills for Pharmacy Calculations - Fractions, Decimals, Percentages
Pharmacy Calculations Tricks and Tips to Master the Math. PEBC, EE, MCQ, FPGEE, KAPS, NAPLEX, NCLEX
Комментарии
 0:16:18
0:16:18
 0:17:52
0:17:52
 0:10:07
0:10:07
 0:37:10
0:37:10
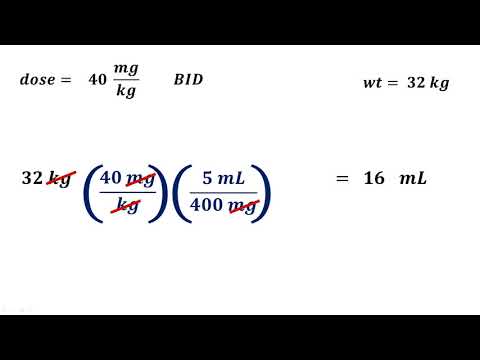 0:07:58
0:07:58
 0:06:05
0:06:05
 0:17:38
0:17:38
 0:10:35
0:10:35
 0:06:50
0:06:50
 0:11:51
0:11:51
 0:08:15
0:08:15
 0:13:47
0:13:47
 0:09:33
0:09:33
 0:21:55
0:21:55
 0:10:33
0:10:33
 0:06:16
0:06:16
 0:20:44
0:20:44
 0:09:03
0:09:03
 2:32:29
2:32:29
 0:00:21
0:00:21
 0:00:11
0:00:11
 0:06:28
0:06:28
 0:22:20
0:22:20
 0:24:31
0:24:31