filmov
tv
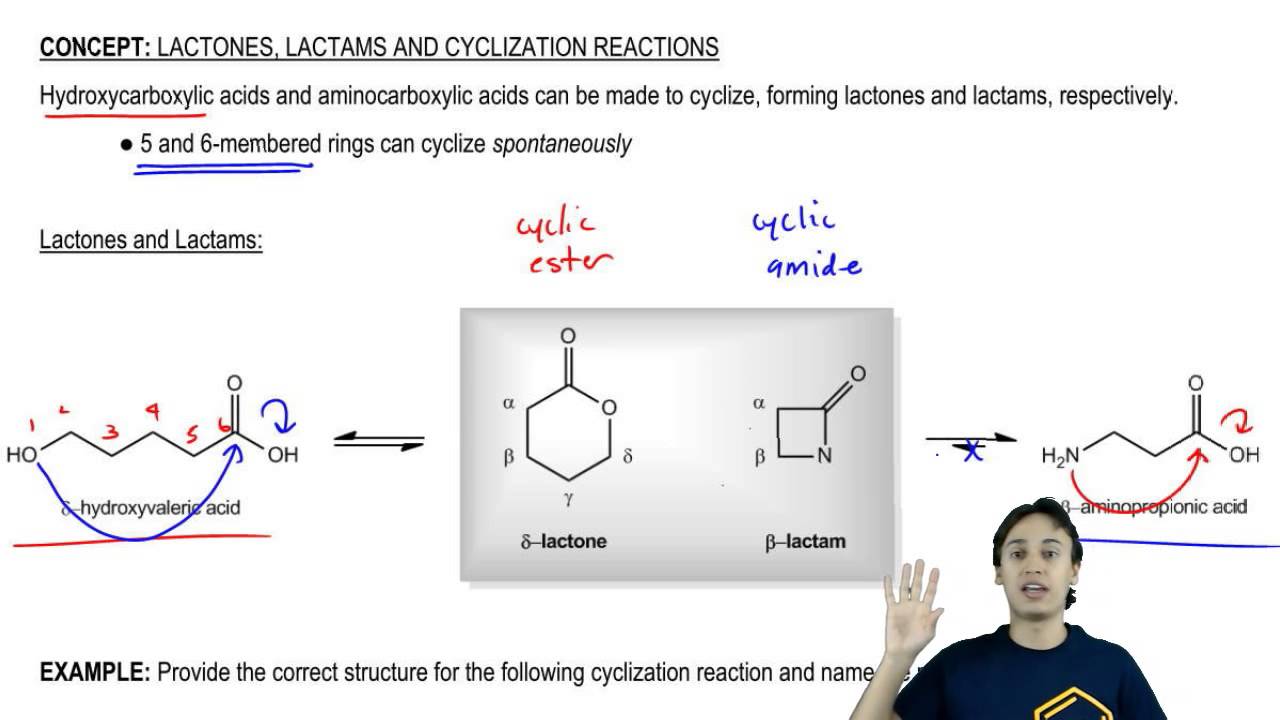
Lactones and Lactams
Показать описание
Explore Channels, available in Pearson+, and access thousands of videos with bite-sized lessons in multiple college courses. The videos are hand-picked, and led by experts, to help you learn faster and easier. Understand tricky concepts, quiz yourself with practice questions, download worksheets to follow along with the lessons and stay on top of your studies. Plus, experts are available for Q&A. Whether you want it paired with your eTextbook in Pearson+, or watch videos on your own time to go from "huh?" to "aha!" in class, Channels is available to you anytime you need it. It's reimagined learning, designed for your learning style.
---------------
----------------
SOCIAL:
----------------
#PearsonPlus #ThisIsYouLearning #studyvideo #StudyHelp #studying #studytutorial #eTextbooks #StudyToolsForLess #MoreBooksFewerBucks #BingeWatchGoodGrades
---------------
----------------
SOCIAL:
----------------
#PearsonPlus #ThisIsYouLearning #studyvideo #StudyHelp #studying #studytutorial #eTextbooks #StudyToolsForLess #MoreBooksFewerBucks #BingeWatchGoodGrades
Комментарии