filmov
tv
The Van't Hoff Factor

Показать описание
This video explains what is and how to determine the van't hoff factor of a reaction.
The Van't Hoff Factor
Molar Mass From Osmotic Pressure - Molarity & Van't Hoff Factor - Chemistry Problems
Van't Hoff Factors
Van't hoff factor and its significance
Finding the ideal Van't Hoff factor
Solutions 08 I Van't Hoff Factor and Abnormal Molar Masses - Most Important Concept IIT JEE/NEE...
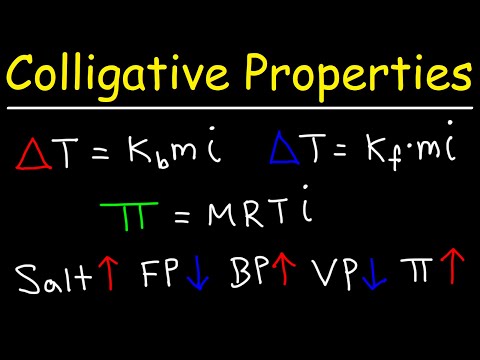
Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure
What is Van't Hoff Factor | Class 12 | Chemistry | Alakh Pandey Sir | @AlakhSirHighlights
13.1 Introduction to Colligative Properties, the van't Hoff factor, and Molality
Trick to Find Van'thoff Factor i | Solutions chapter | Class 12 chemistry.
Calculation of dissociation factor | Van't Hoff factor
Van't Hoff Factor explained in 5 min | Unacademy Atoms | Paaras Thakur
Vant Hoff factor |Class 12- Chapter 2| chemistry
ALEKS: Calculating and using the van't Hoff factor for electrolytes
Finding the Van't Hoff Factor | Learn Chemistry with Ma'am Cess
Solutions | Class 12 (L4) | van't Hoff factor | Henry's law |Azeotropes
Solutions Chemistry Class 12 - Calculation of Van't Hoff Factor | NEET 2023 PYQ - 4 Marks in 4 ...
Abnormal Molar Masses | van't Hoff Factor | Degree of Dissociation (Solution Chemistry 12th)
ALEKS - Calculating and using the van't Hoff factor for electrolytes (2 of 2)
ALEKS - Calculating and using the van't Hoff factor for electrolytes (1 of 2)
Solutions(Vant Hoff Factor) | NEET/JEE/AIIMS 2019 Chemistry (L-14) | by Arvind Arora
Van’t Hoff factor Numericals | Class 12 Chemistry | Chapter Solutions Numerical | PYQ
VAN'T HOFF FACTOR || solution and colligative properties.
Solutions 08 | Van't Hoff Factor (Practice Session) | Class 12th/CUET
Комментарии
 0:04:22
0:04:22
 0:10:59
0:10:59
 0:02:28
0:02:28
 0:15:24
0:15:24
 0:00:52
0:00:52
 1:23:43
1:23:43
 0:25:23
0:25:23
 0:01:41
0:01:41
 0:16:56
0:16:56
 0:13:25
0:13:25
 0:17:03
0:17:03
 0:05:58
0:05:58
 0:07:05
0:07:05
 0:07:31
0:07:31
 0:25:49
0:25:49
 0:38:37
0:38:37
 0:03:05
0:03:05
 0:30:09
0:30:09
 0:06:22
0:06:22
 0:10:36
0:10:36
 0:27:43
0:27:43
 0:26:34
0:26:34
 0:18:03
0:18:03
 1:25:12
1:25:12