filmov
tv
Understanding ORP: Oxidation Reduction Potential | Orenda Whiteboard

Показать описание
Understanding ORP: Oxidation Reduction Potential
So how do we know if chlorine is really doing its job in your swimming pool? How do we know how well chlorine is performing? Free chlorine only tells us how much chlorine is in the water...it neglects things like stabilizer and efficiency. But there's a measurement for chlorine's performance, and it's called Oxidation Reduction Potential (ORP).
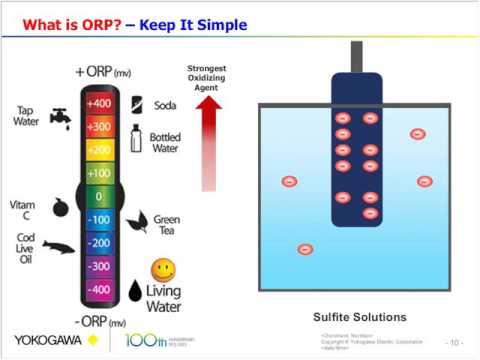
ORP is sometimes called the "conductivity" of water. ORP is measured through electrodes in an automated chemical controller, which measure the rate of electron transfer between the probes, in millivolts (mV). The faster the electron transfer, the more conductive the water, the higher the ORP, and the stronger the chlorine. The higher, the better, and ORP is usually measured on a scale up to 850 or 900 mV.
We ideally want ORP in the high 700s or even 800s, which would be excellent. Most health codes have a minimum of 650mV. If your ORP is below that, the chlorine may not be keeping up with the bather load in your swimming pool. It is slowed down or overwhelmed, you might say.
What is Oxidation? What is Reduction? Are they the same?
Oxidation and reduction are actually opposites. Oxidation is when an oxidizer (like chlorine) steals electrons from an oxidant (like bather waste, or iron). The oxidant loses the electrons and either becomes inert, destroyed or transforms into an oxidized form (for example, iron will become ferrous iron and turn orange or brown, leading to pool stains). Bather waste tends to be destroyed by oxidation, or chemically "burned up" in a manner of speaking.
Oxidation and Reduction are the two sides of a Redox reaction.
Anyway, the electrons taken from the oxidant have a negative charge (e-). That negative charge consequentially REDUCES the valence of the oxidizer. So if you have hypochlorous acid, the strong form of chlorine (HOCl), it would be REDUCED when it pulls electrons from an oxidant like bather waste. It will be REDUCED down to useless chloride ions (Cl-), which can no longer take on more electrons--therefore they can no longer oxidize--and becomes "used up". So when we say chlorine gets "reduced" or "used up", we're talking about reduction.
The acronym to remember is OILRIG. Oxidation is Loss (of electrons), and Reduction is Gain (of electrons, which reduce the valence of the oxidizer).
Oxidants that Reduce Chlorine and lower ORP
We recommend enzymes for handling bather waste, which also uses up chlorine. UV and other secondary systems like Ozone also help tremendously.
ORP and LSI: The Future of Water Management
So how do we know if chlorine is really doing its job in your swimming pool? How do we know how well chlorine is performing? Free chlorine only tells us how much chlorine is in the water...it neglects things like stabilizer and efficiency. But there's a measurement for chlorine's performance, and it's called Oxidation Reduction Potential (ORP).
ORP is sometimes called the "conductivity" of water. ORP is measured through electrodes in an automated chemical controller, which measure the rate of electron transfer between the probes, in millivolts (mV). The faster the electron transfer, the more conductive the water, the higher the ORP, and the stronger the chlorine. The higher, the better, and ORP is usually measured on a scale up to 850 or 900 mV.
We ideally want ORP in the high 700s or even 800s, which would be excellent. Most health codes have a minimum of 650mV. If your ORP is below that, the chlorine may not be keeping up with the bather load in your swimming pool. It is slowed down or overwhelmed, you might say.
What is Oxidation? What is Reduction? Are they the same?
Oxidation and reduction are actually opposites. Oxidation is when an oxidizer (like chlorine) steals electrons from an oxidant (like bather waste, or iron). The oxidant loses the electrons and either becomes inert, destroyed or transforms into an oxidized form (for example, iron will become ferrous iron and turn orange or brown, leading to pool stains). Bather waste tends to be destroyed by oxidation, or chemically "burned up" in a manner of speaking.
Oxidation and Reduction are the two sides of a Redox reaction.
Anyway, the electrons taken from the oxidant have a negative charge (e-). That negative charge consequentially REDUCES the valence of the oxidizer. So if you have hypochlorous acid, the strong form of chlorine (HOCl), it would be REDUCED when it pulls electrons from an oxidant like bather waste. It will be REDUCED down to useless chloride ions (Cl-), which can no longer take on more electrons--therefore they can no longer oxidize--and becomes "used up". So when we say chlorine gets "reduced" or "used up", we're talking about reduction.
The acronym to remember is OILRIG. Oxidation is Loss (of electrons), and Reduction is Gain (of electrons, which reduce the valence of the oxidizer).
Oxidants that Reduce Chlorine and lower ORP
We recommend enzymes for handling bather waste, which also uses up chlorine. UV and other secondary systems like Ozone also help tremendously.
ORP and LSI: The Future of Water Management
Комментарии
 0:01:43
0:01:43
 0:46:51
0:46:51
 0:01:35
0:01:35
 0:44:45
0:44:45
 0:01:57
0:01:57
 0:05:07
0:05:07
 0:08:18
0:08:18
 0:01:33
0:01:33
 0:01:33
0:01:33
 0:42:30
0:42:30
 0:48:11
0:48:11
 0:01:01
0:01:01
 0:06:44
0:06:44
 0:02:56
0:02:56
 0:42:58
0:42:58
 0:01:35
0:01:35
 0:00:27
0:00:27
 0:01:14
0:01:14
 0:02:23
0:02:23
 0:02:44
0:02:44
 0:05:34
0:05:34
 0:07:14
0:07:14
 0:01:00
0:01:00
 0:04:01
0:04:01