filmov
tv
Clinical Trials Overview: Phrases and Phases of a Clinical Trials

Показать описание
Dr. Hilary Vernon leads an informative discussion about the basics of clinical trials.
Clinical Trials Overview: Phrases and Phases of a Clinical Trials
Understanding Clinical Trials
The Four Phases of Clinical Trials | Diversity in Clinical Trials | AKF
Clinical Trial Lifecycle Overview #clinicalresearch #pharmacovigilance #clinicaltrials
Clinical Trials & ClinicalTrials.gov explained
Introduction to Clinical Study Design: Where to Start Part 1
Clinical Trial
Clinical Trials
Master 100 Medical Phrases in Just 10 Minutes! 🩺🗣
Clinical Trials 101
The Clinical Trial Journey
Introduction To Clinical Research
Clinical Trials: Words & Phases
What Are Clinical Trial Phases?
Clinical Trials 101
Clinical Trial - What You Need to Know to ACE Your Interview or Exam
Phrases & Idioms in English #shorts #idioms #phrases
Phase 1 Clinical Trial | Words to Know, NCI Dictionary of Cancer Terms
How to interpret clinical trial data – Examples from recent clinical trials
Understanding Your Clinical Trial Results
A Brief Overview of Clinical Trials
chapter 3: TERMS AND DEFINITIONS IN CLINICAL RESEARCH
Clinical Research Site Operations Basic Overview
Clinical trials - Medical device and drug development
Комментарии
 1:01:14
1:01:14
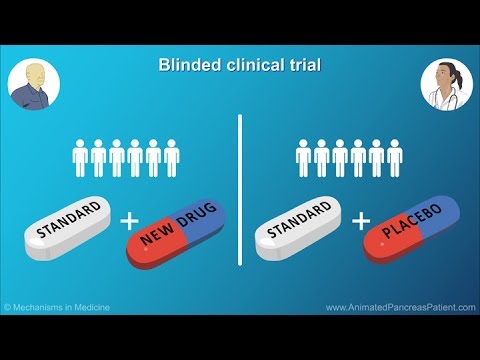 0:06:59
0:06:59
 0:03:54
0:03:54
 0:01:59
0:01:59
 0:04:01
0:04:01
 0:16:00
0:16:00
 0:04:24
0:04:24
 0:02:25
0:02:25
 0:09:01
0:09:01
 0:13:37
0:13:37
 0:02:46
0:02:46
 0:17:55
0:17:55
 0:04:38
0:04:38
 0:01:49
0:01:49
 0:04:57
0:04:57
 0:06:24
0:06:24
 0:00:06
0:00:06
 0:00:50
0:00:50
 0:37:30
0:37:30
 0:06:00
0:06:00
 0:03:30
0:03:30
 0:04:25
0:04:25
 0:19:39
0:19:39
 0:06:30
0:06:30