filmov
tv
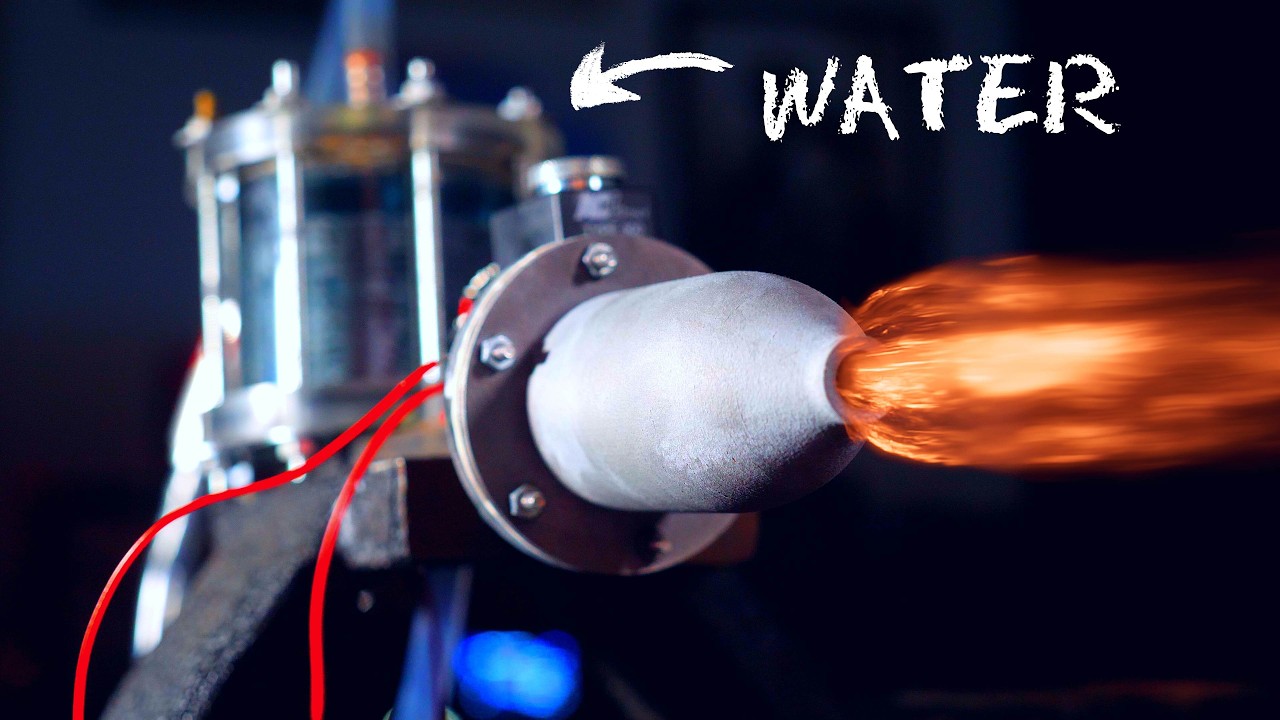
ROCKET that LITERALLY BURNS WATER as FUEL
Показать описание
3D Models
Materials
Machines
#engine #rocket #3dprinting
ROCKET that LITERALLY BURNS WATER as FUEL
Why rockets eject half a million gallons of water
SHE PULLED THE SWORD OUT OF THE STONE RIGHT IN FRONT OF ME IN DISNEY WORLD
The Best Performing (and most dangerous) Chemical Rocket Ever Tested: Rocketdyne Tripropellant
Why don't rocket engines melt? How engineers keep engines cool
How Much Do Rockets Pollute? Are They Bad For Our Air?
What's Going On In This SpaceX Rocket Video?
SpaceX's Water Landing Reveals Rocket 'Secrets' (or, What We Learned from CRS-16)
Defying Atmosphere - How Rocket Engines Get Tested In A Vacuum Before Going To Space
Green screening myself into Station 19's Rocket Water Heater crap show.
Rocket Scientists Answer Questions From Twitter | Tech Support | WIRED
Rocket Science: Explained (Also - NASA found water on the moon so let’s chat about it)
The rocket motor that broke me
Model Rocket Engine In A Vacuum Chamber - 4K Slow Motion - will it burn? - Rockets (S1 • E3)
Rocket Monday Ep67 (ARCA Steam Rocket Explained)
Ethanol/oxygen liquid fuel rocket engine test compilation
Turn Empty Water Bottles Into Alcohol Fueled Rockets
How Fire Burns in Space
Liberator ROCKET Heater -Pellets w/ NO ELECTRICITY !!!
Engineering Connections (Richard Hammond) - Space Shuttle | Science Documentary | Reel Truth Science
What keeps everyone safe when rockets fail? Why did the failed Falcon 9 rocket land in the ocean?
I Built The First LAMINAR FLOW ROCKET ENGINE
Rocket heater pulsing burn shakes the room / heating German V2 A4 rocket rudder part
What it's like inside rocket engines! Viking 2 and Vulcain 1!
Комментарии
 0:19:00
0:19:00
 0:04:33
0:04:33
 0:00:41
0:00:41
 0:53:44
0:53:44
 0:26:31
0:26:31
 0:55:46
0:55:46
 0:12:40
0:12:40
 0:14:16
0:14:16
 0:11:21
0:11:21
 0:03:16
0:03:16
 0:14:32
0:14:32
 0:08:25
0:08:25
 0:25:11
0:25:11
 0:05:30
0:05:30
 0:09:51
0:09:51
 0:00:42
0:00:42
 0:02:12
0:02:12
 0:03:02
0:03:02
 0:17:57
0:17:57
 0:50:00
0:50:00
 0:28:10
0:28:10
 0:15:51
0:15:51
 0:02:36
0:02:36
 0:26:01
0:26:01