filmov
tv
Understanding the NPK Ratio of Fertilizers (With Examples)

Показать описание
Follow us:
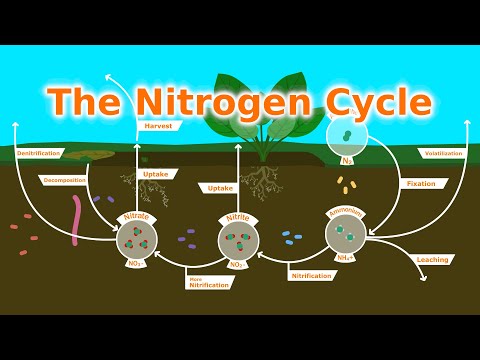
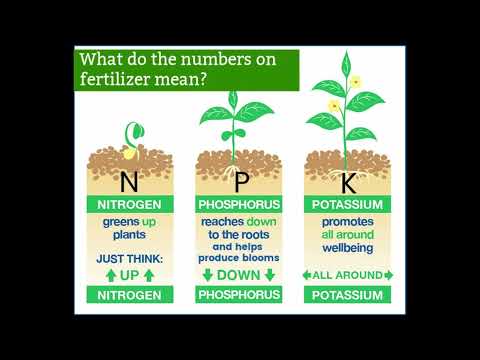
Note: The NPK ratio is the fertilizer analysis that tells you information about three key nutrients: nitrogen, phosphorus, and potassium. It is usually written in large bold letters on the packaging.
Q1. In package whose NPK ratio is 33-10-5, what percentage of the product contains nitrogen, P2O5, and K2O? If the package weighs 40 lbs, how much of each nutrient does it contain by weight.
Note: If you're asked for the percentage of elemental P and K, the calculation changes.
• Firstly, the molecular weight of P2O5 is 142 grams/mol. The portion of the molecule that is P only is 62 grams/mol. Therefore, P represents 62/142=0.4366 or 0.44 (44%) of the molecule.
• Similarly, K2O has a molecular weight of 94 grams/mol. The portion of the molecule that is potassium is 78 grams/mol. Hence, potassium only represents 78/94=0.8298 or 0.83 (83%) of the molecule.
Q2.
a) For the 50 lb. bag of fertilizer depicted below, state the percent of:
N = ___%
P2O5 = ___%
P = ___%
K2O = ___%
K = ___%
Filler = ___%
Total = ___%
b) Calculate the weight of each nutrient in pounds (lb.):
N = ___ lb.
P2O5 = ___ lb.
P = ___ lb.
K2O = ___ lb.
K = ___ lb.
Filler = ___ lb.
Total = ___ lb.
Комментарии
 0:01:26
0:01:26
 0:03:34
0:03:34
 0:07:48
0:07:48
 0:09:48
0:09:48
 0:02:16
0:02:16
 0:03:35
0:03:35
 0:11:34
0:11:34
 0:04:23
0:04:23
 0:02:01
0:02:01
 0:16:42
0:16:42
 0:12:28
0:12:28
 0:07:11
0:07:11
 0:09:57
0:09:57
 0:11:18
0:11:18
 0:09:28
0:09:28
 0:00:24
0:00:24
 0:04:30
0:04:30
 0:15:33
0:15:33
 0:03:05
0:03:05
 0:04:28
0:04:28
 0:05:37
0:05:37
 0:11:41
0:11:41
 0:02:13
0:02:13
 0:11:33
0:11:33