filmov
tv
New Drug Discovery and Development (Overview) - Part 1 | Dr. Shikha Parmar

Показать описание
New Drug Discovery and Development (Overview) by Dr. Shikha Parmar
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes pre-clinical research on microorganisms and animals, filing for regulatory statutes, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug.
(1) The first step in the drug development process involves discovery work. This is where drug development companies choose a molecule, such as a gene or protein, to target with a drug.
(2) The next step in the drug development process is preclinical testing, which in itself is divided into two subcomponents: in vitro and in vivo testing. In vitro testing examines the drug molecules' interactions in test tubes and within the lab setting.
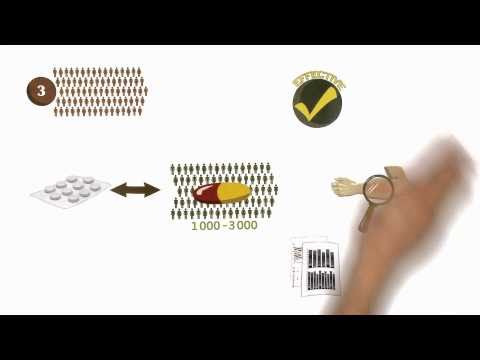
(3) The third step involves submitting an Investigational New Drug Application to the FDA prior to beginning human clinical trials.
(4)Clinical trials
Phase I trials, usually in healthy volunteers, determine safety and dosing.
Phase II trials are used to get an initial reading of efficacy and further explore safety in small numbers of patients having the disease targeted by the NCE.
Phase III trials are large, pivotal trials to determine safety and efficacy in sufficiently large numbers of patients with the targeted disease. If safety and efficacy are adequately proved, clinical testing may stop at this step and the NCE advances to the new drug application (NDA) stage.
Phase IV trials are post-approval trials that are sometimes a condition attached by the FDA, also called post-market surveillance studies.
Description Source: Wikipedia
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes pre-clinical research on microorganisms and animals, filing for regulatory statutes, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug.
(1) The first step in the drug development process involves discovery work. This is where drug development companies choose a molecule, such as a gene or protein, to target with a drug.
(2) The next step in the drug development process is preclinical testing, which in itself is divided into two subcomponents: in vitro and in vivo testing. In vitro testing examines the drug molecules' interactions in test tubes and within the lab setting.
(3) The third step involves submitting an Investigational New Drug Application to the FDA prior to beginning human clinical trials.
(4)Clinical trials
Phase I trials, usually in healthy volunteers, determine safety and dosing.
Phase II trials are used to get an initial reading of efficacy and further explore safety in small numbers of patients having the disease targeted by the NCE.
Phase III trials are large, pivotal trials to determine safety and efficacy in sufficiently large numbers of patients with the targeted disease. If safety and efficacy are adequately proved, clinical testing may stop at this step and the NCE advances to the new drug application (NDA) stage.
Phase IV trials are post-approval trials that are sometimes a condition attached by the FDA, also called post-market surveillance studies.
Description Source: Wikipedia
Комментарии
 0:07:22
0:07:22
 0:07:50
0:07:50
 0:02:52
0:02:52
 0:04:41
0:04:41
 0:20:27
0:20:27
 0:14:17
0:14:17
 0:04:33
0:04:33
 0:04:15
0:04:15
 0:00:54
0:00:54
 0:01:00
0:01:00
 0:21:51
0:21:51
 0:02:00
0:02:00
 0:52:43
0:52:43
 0:04:33
0:04:33
 0:02:19
0:02:19
 0:06:47
0:06:47
 0:19:03
0:19:03
 1:26:32
1:26:32
 0:25:21
0:25:21
 0:03:11
0:03:11
 0:28:04
0:28:04
 0:22:49
0:22:49
 0:15:40
0:15:40
 0:49:31
0:49:31