filmov
tv
8.27g | How to find the hybridization of the central atom in ClOF2+

Показать описание
Identify the hybridization of the central atom in each of the following molecules and ions that contain multiple bonds:
ClOF2+ (Cl is the central atom)
OpenStax™ is a registered trademark, which was not involved in the production of, and does not endorse, this product.
If you don't have the OpenStax™ "Chemistry: Atoms First" textbook, here is a link in which you can download it for FREE!
SUBSCRIBE if you'd like to see more solutions for your textbook!
Want us as your private tutor? Get started with your FREE initial assessment!
#Hybridization #ValenceBondTheory #OpenStaxChemistry
ClOF2+ (Cl is the central atom)
OpenStax™ is a registered trademark, which was not involved in the production of, and does not endorse, this product.
If you don't have the OpenStax™ "Chemistry: Atoms First" textbook, here is a link in which you can download it for FREE!
SUBSCRIBE if you'd like to see more solutions for your textbook!
Want us as your private tutor? Get started with your FREE initial assessment!
#Hybridization #ValenceBondTheory #OpenStaxChemistry
How many moles are in 27.0 g of H2O ?
A nutritionist explains an easy way to understand how much sugar you're eating daily
How to Convert Grams to Kilograms
Drawing Up A Syringe: Semaglutide Instructions
What Needle Should I Use for TRT? (Testosterone Replacement Therapy)
HYDRAULIC PRESS VS BALL BEARINGS! Which will EXPLODE first?
The weight of oxygen required to completely react with 27g of aluminium is
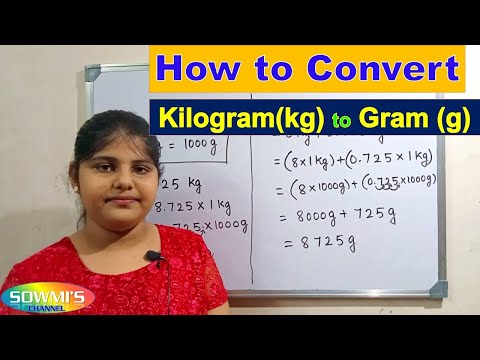
Conversion of kg to g | how to convert kilogram to gram | kilogram into gram
The Exact Insulin Syringes I Use To Pin My TRT
27g of Al react with how much mass of O2 to produce Al2O3?
How to Increase and Decrease in a Ratio || Maths Lit
How to read a syringe
How to Inject Testosterone for Men: From Start to Finish
How to use the BD SafetyGlide™ insulin syringe with an 8mm needle for diabetes management
`27 g` of `Al` will react completely with `g` of `O_(2)`
GCSE Maths - How to Calculate Percentage Change (Increase or Decrease) #94
23G Or 25G Needle - Which Should You Use For Testosterone Injections?
Tamasha Dekho 😂 IITian Rocks Relatives Shock 😂😂😂 #JEEShorts #JEE #Shorts
How to Convert g/cm3 to kg/m3 (And NEVER BE WRONG AGAIN)
How much sugar is in your drink? - Medical Minute
Insulin syringes for testosterone injections?
How 5 gram creatine is equal 1.3 teaspoon for muscle growth
Chemistry ka JAAADU🪄 #shorts #experiments || PW Pathshala
THIS BURNED for 36 MINUTES! - INSANE RESULTS - 27g Esbit VS 27g FireDragon Fuel Tablets
Комментарии
 0:03:14
0:03:14
 0:00:44
0:00:44
 0:01:56
0:01:56
 0:01:02
0:01:02
 0:08:04
0:08:04
 0:01:19
0:01:19
 0:01:19
0:01:19
 0:06:57
0:06:57
 0:12:54
0:12:54
 0:02:02
0:02:02
 0:01:55
0:01:55
 0:05:12
0:05:12
 0:05:29
0:05:29
 0:02:40
0:02:40
 0:01:57
0:01:57
 0:04:07
0:04:07
 0:07:39
0:07:39
 0:00:13
0:00:13
 0:02:21
0:02:21
 0:02:43
0:02:43
 0:01:12
0:01:12
 0:02:20
0:02:20
 0:00:55
0:00:55
 0:18:07
0:18:07