filmov
tv
EGFR Epidermal Growth Factor Receptor (a transglutaminase substrate) and cancer by Kevin Ahern

Показать описание
Kevin Ahern
------------------
HUMAN TRANSGLUTAMINASE (TG2 or tTG) & CANCER & EPIDERMAL GROWTH FACTOR RECEPTOR (EGFR)
-------------------------------------------------------------------------------------------
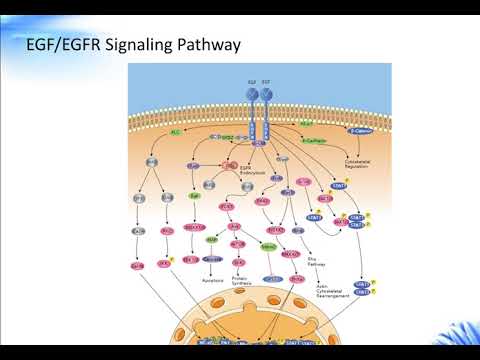
The EGF receptor is a cell surface receptor tyrosine kinase that is expressed in a variety of normal cell lineages. Upon binding ligand, the EGF receptor becomes activated and initiates a highly regulated series of signaling events, which direct changes in gene transcription and various cellular responses that are vital for proper development and tissue homeostasis (Antonyak 2009)
Post-translational modification (PTM) is an important mechanism in modulating a protein’s structure and can lead to substantial diversity in biological function. The development of novel and more sensitive techniques has led to more proteins identified as tissue transglutaminase (TG2) substrates. Many of these substrate proteins play a role in cell signaling.... (LAI 2017).
EGF receptor (EGFR) is a tissue transglutaminase substrate (TRANSDAB)
Research demonstrating TG2’s inhibitory effect on EGF signalling has additionally established that the extracellular domain of EGFR is a Tissue transglutaminase (TG2) substrate (MARUKO 2009)
Tissue transglutaminase plays a key part in the progression of cancer and survival (KATT 2018).
Tissue transglutaminase (tTG) promotes the growth and survival of several different cancer cell types, outcomes that are largely thought to be dependent on its acyltransferase (protein crosslinking) activity (Zhang 2013).
It is notable that cytoplasmic Tissue transglutaminase (TG2)-mediated transamidase activity participates in EGF receptor signalling.... (ALGARNI 2018)
EGF receptor (EGFR) signaling in human cancers elicits changes in protein-expression patterns that are crucial for potentiating tumor growth. Identifying those proteins with expression regulated by the EGFR and determining how they contribute to malignancy is fundamental for the development of more effective strategies to treat cancer. Here, we show that tissue transglutaminase (tTG) is one such protein (Antonyak 2010)
In addition to its physiological functions, the EGF receptor has also been intimately associated with oncogenesis. Enhanced EGF receptor signaling, as a result of receptor overexpression and/or an autocrine stimulation of the receptor, is a hallmark of a variety of human tumors...These findings... strongly implicate EGF receptor signaling in cancer development (Antonyak 2009)
The EGF/EGF receptor (EGFR) pathway, which is often hyperactivated in human malignancies, upregulated Tissue transglutaminase (TG2) expression in cervical and breast epithelial cancer cells... EGF signaling effect ... required Src activity and the formation of ternary cytoplasmic complexes between Src and keratin-19, mediated by TG2. Much like with retinoids, the EGF signaling through Ras and JNK was required for targeting TG2 to the leading edges of the cells and activating transamidation (Nurminskaya 2012)
EGFR activity, which is frequently increased in human malignant cells, increases TG2 expression in cervical, breast, and lung epithelial cancer cells. Moreover, induction of TG2 expression and TG2-dependent transamidation are essential for EGF-mediated migration, invasion, and anchorage-independent cancer cell growth. TG2-induced Src expression is associated with transamidation-dependent formation of cytoplasmic ternary complexes of Src, TG2, and keratin 19. Thus cytoplasmic TG2 is a novel mediator of EGF/EGFR-induced signaling and oncogenesis in epithelial cancer cells that involves TG2 transamidation-dependent and -independent actions (Eckert 2014).
------------------
HUMAN TRANSGLUTAMINASE (TG2 or tTG) & CANCER & EPIDERMAL GROWTH FACTOR RECEPTOR (EGFR)
-------------------------------------------------------------------------------------------
The EGF receptor is a cell surface receptor tyrosine kinase that is expressed in a variety of normal cell lineages. Upon binding ligand, the EGF receptor becomes activated and initiates a highly regulated series of signaling events, which direct changes in gene transcription and various cellular responses that are vital for proper development and tissue homeostasis (Antonyak 2009)
Post-translational modification (PTM) is an important mechanism in modulating a protein’s structure and can lead to substantial diversity in biological function. The development of novel and more sensitive techniques has led to more proteins identified as tissue transglutaminase (TG2) substrates. Many of these substrate proteins play a role in cell signaling.... (LAI 2017).
EGF receptor (EGFR) is a tissue transglutaminase substrate (TRANSDAB)
Research demonstrating TG2’s inhibitory effect on EGF signalling has additionally established that the extracellular domain of EGFR is a Tissue transglutaminase (TG2) substrate (MARUKO 2009)
Tissue transglutaminase plays a key part in the progression of cancer and survival (KATT 2018).
Tissue transglutaminase (tTG) promotes the growth and survival of several different cancer cell types, outcomes that are largely thought to be dependent on its acyltransferase (protein crosslinking) activity (Zhang 2013).
It is notable that cytoplasmic Tissue transglutaminase (TG2)-mediated transamidase activity participates in EGF receptor signalling.... (ALGARNI 2018)
EGF receptor (EGFR) signaling in human cancers elicits changes in protein-expression patterns that are crucial for potentiating tumor growth. Identifying those proteins with expression regulated by the EGFR and determining how they contribute to malignancy is fundamental for the development of more effective strategies to treat cancer. Here, we show that tissue transglutaminase (tTG) is one such protein (Antonyak 2010)
In addition to its physiological functions, the EGF receptor has also been intimately associated with oncogenesis. Enhanced EGF receptor signaling, as a result of receptor overexpression and/or an autocrine stimulation of the receptor, is a hallmark of a variety of human tumors...These findings... strongly implicate EGF receptor signaling in cancer development (Antonyak 2009)
The EGF/EGF receptor (EGFR) pathway, which is often hyperactivated in human malignancies, upregulated Tissue transglutaminase (TG2) expression in cervical and breast epithelial cancer cells... EGF signaling effect ... required Src activity and the formation of ternary cytoplasmic complexes between Src and keratin-19, mediated by TG2. Much like with retinoids, the EGF signaling through Ras and JNK was required for targeting TG2 to the leading edges of the cells and activating transamidation (Nurminskaya 2012)
EGFR activity, which is frequently increased in human malignant cells, increases TG2 expression in cervical, breast, and lung epithelial cancer cells. Moreover, induction of TG2 expression and TG2-dependent transamidation are essential for EGF-mediated migration, invasion, and anchorage-independent cancer cell growth. TG2-induced Src expression is associated with transamidation-dependent formation of cytoplasmic ternary complexes of Src, TG2, and keratin 19. Thus cytoplasmic TG2 is a novel mediator of EGF/EGFR-induced signaling and oncogenesis in epithelial cancer cells that involves TG2 transamidation-dependent and -independent actions (Eckert 2014).
Комментарии
 0:03:47
0:03:47
 0:02:04
0:02:04
 0:03:02
0:03:02
 0:02:06
0:02:06
 0:08:30
0:08:30
 0:01:57
0:01:57
 0:07:17
0:07:17
 0:00:42
0:00:42
 0:28:12
0:28:12
 0:30:22
0:30:22
 0:01:59
0:01:59
 0:15:47
0:15:47
 0:15:53
0:15:53
 0:01:16
0:01:16
 0:03:07
0:03:07
 0:04:33
0:04:33
 0:04:07
0:04:07
 0:01:00
0:01:00
 0:14:17
0:14:17
 0:00:43
0:00:43
 0:19:05
0:19:05
 0:00:17
0:00:17
 0:00:10
0:00:10
 0:02:16
0:02:16