filmov
tv
Finding Mass of Fluorine in 24.6g SnF₂ Toothpaste Ingredient Explained!

Показать описание
Tin(II) Fluoride in Toothpaste: Dive into the chemistry of Tin(II) Fluoride commonly used in toothpaste to prevent tooth decay. Understand molar mass calculations stoichiometry and determine the fluoride content in a given compound
Textbook Question: Tin(II) fluoride is often added to toothpaste as an ingredient to prevent tooth decay. What is the mass of F in grams in 24.6 g of the compound?
StudySoup delivers curated study tools tailored for university students, empowering them to grasp more in less time. Our content zeroes in on key concepts, presented in a format that resonates best with student learning styles. Dive into our carefully crafted video content that aligns with general education and core curriculum, setting students up for exam success. Plus, for those navigating the intricacies of STEM courses, StudySoup offers invaluable tools crafted to support your higher education journey.
Socials
 0:00:15
0:00:15
 0:01:47
0:01:47
 0:04:52
0:04:52
 0:04:07
0:04:07
 0:00:48
0:00:48
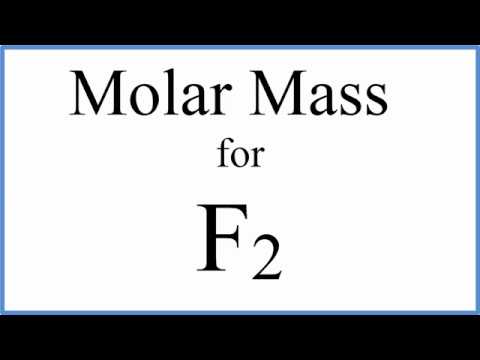 0:00:59
0:00:59
 0:01:39
0:01:39
 0:02:38
0:02:38
 0:00:54
0:00:54
 0:05:54
0:05:54
 0:07:16
0:07:16
 0:00:49
0:00:49
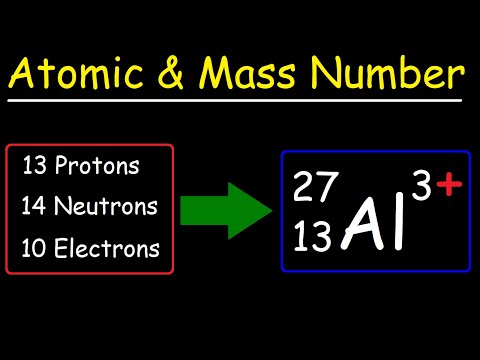 0:11:41
0:11:41
 0:01:24
0:01:24
 0:03:50
0:03:50
 0:13:12
0:13:12
 0:04:16
0:04:16
 0:01:03
0:01:03
 0:02:29
0:02:29
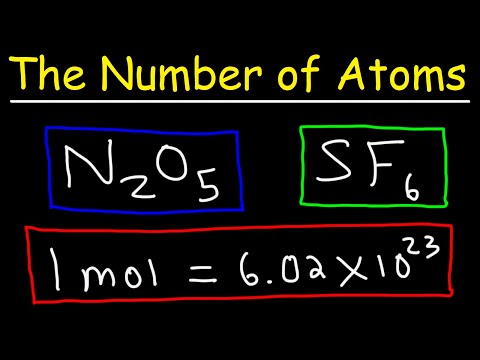 0:13:48
0:13:48
 0:00:14
0:00:14
 0:00:27
0:00:27
 0:00:33
0:00:33
 0:04:30
0:04:30