filmov
tv
Molar Gas Volume: Stoichiometry With Gases

Показать описание
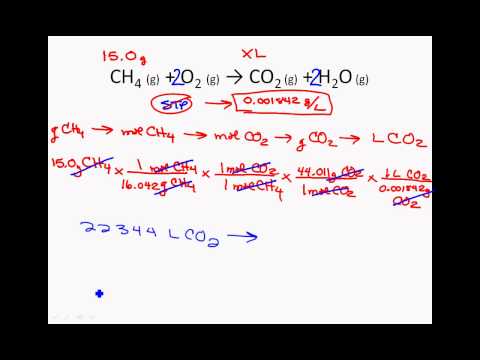
We know a lot about ideal gases, including how to use all of the ideal gas laws. But we haven't talked much about how to do stoichiometry with gases. As it happens, we can do stoichiometry with gases not just using molar quantities, but also by using volumes. Find out how!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Molar Gas Volume: Stoichiometry With Gases
Molar Volume Of Gas | Chemical Calculations | Chemistry | FuseSchool
Gas Stoichiometry Problems
Converting Between Moles and Liters of a Gas at STP
GCSE Chemistry - How to Find the Volume of a Gas
Molar Volume - Stoichiometry 007
Quantitative Aspects of Chemical Change: Molar Volume
Molar Volume - Stoichiometry 008
Solution Stoichiometry - Finding Molarity, Mass & Volume
Gas Density and Stoichiometry
Gases V - Molar Volume & Gas Stoichiometry
Gas Law Stoichiometry - Volume-Volume Problem
Gas Stoichiometry: Volume-Volume
4 molar volume stoichiometry
How to calculate volume of a gas using molar gas volume 24dm^3/mole
GCSE Chemistry Revision 'Using Gas Volumes' (Triple)
Gas Stoichiometry - Example 1 - at STP
Molar Volume - Stoichiometry 006
GCSE Chemistry - Moles, Concentration & Volume Calculations
Gas Volume Stoichiometry | Unit Conversions
lec#3 Molar Volume Of Gas || Molar Gas Volume: Stoichiometry With Gases || 11th class new book 2024
How to use Molar Volume in Stoichiometry Calculations involving Gases
Step by Step Gas Stoichiometry - Final Exam Review
Stoichiometry with Volume of a Gas #chemistry #science #homework #shorts #short
Комментарии
 0:05:10
0:05:10
 0:03:23
0:03:23
 0:31:20
0:31:20
 0:12:43
0:12:43
 0:06:58
0:06:58
 0:03:40
0:03:40
 0:10:36
0:10:36
 0:02:45
0:02:45
 0:23:11
0:23:11
 0:11:03
0:11:03
 0:06:26
0:06:26
 0:06:18
0:06:18
 0:02:36
0:02:36
 0:03:35
0:03:35
 0:05:19
0:05:19
 0:03:33
0:03:33
 0:01:36
0:01:36
 0:03:16
0:03:16
 0:06:04
0:06:04
 0:02:50
0:02:50
 0:03:28
0:03:28
 0:08:05
0:08:05
 0:14:56
0:14:56
 0:01:01
0:01:01