filmov
tv
Keynote Presentation: Modulation of the CSPG Receptor PTPσ to Enhance Neurorepair

Показать описание
Presented By: Marc DePaul, PhD
Speaker Biography: Dr. Marc DePaul is the Director of Research at NervGen Pharma, a clinical stage biotech company dedicated to developing innovative treatments for nervous system repair. With over 11 years of experience in the biotech industry as a regenerative medicine scientist or consultant, his research strives to identify and develop therapeutics to enhance nervous system repair in disorders and injuries by promoting plasticity, regeneration, and remyelination. He received his Ph.D. in Neurosciences and studied as a post-doc at Case Western Reserve University, where he helped develop the technology NervGen’s platform is founded on.
Webinar: Keynote Presentation: Modulation of the CSPG Receptor PTPσ to Enhance Neurorepair
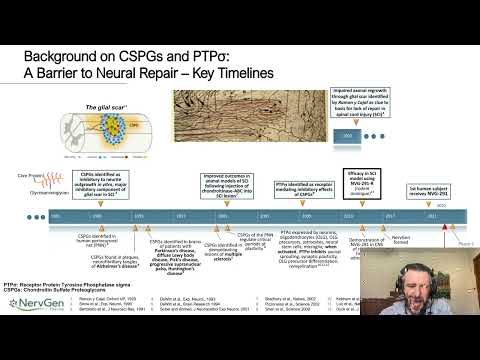
Webinar Abstract: There are currently no approved therapeutics to promote neural repair following central nervous system (CNS) damage. Groundbreaking studies in neurotrauma and disease found that a family of extracellular matrix (ECM) molecules known as chondroitin sulfate proteoglycans (CSPGs) are increased at sites of CNS damage and inhibit endogenous repair mechanisms. The inhibitory effects of CSPGs are mediated through protein tyrosine phosphatase sigma (PTPσ), a potent CSPG receptor. NervGen has developed a novel, first-in-class PTPσ modulator, known as NVG-291, for treatment of nervous system damage due to trauma or disease. NVG-291 is a systemically administered, blood-brain barrier penetrating peptide currently undergoing a Phase 1 trial in healthy volunteers to investigate safety, tolerability and pharmacokinetics.
NVG-291 modulates the cellular response and impact of CSPGs on neural repair, regeneration, remyelination, plasticity and other processes within the central and peripheral nervous systems. Following nervous system damage, CSPGs are upregulated within the forming scar around areas of damage, where they inhibit regeneration of injured axons. In non-damaged CNS tissue, CSPGs are a key component of the perineuronal net (PNN), an ECM structure surrounding synapses, where they help maintain synaptic connections by restricting axonal sprouting and plasticity. However, the presence of PNNs in an injured environment inhibits synaptic and axonal plasticity, which could otherwise facilitate recovery through the generation of novel circuits to compensate for injured or dysfunctional pathways. NVG-291 modulation of the CSPG receptor, PTPσ, promotes injured axons to regenerate through areas of damage, and non-injured axons away from damage to sprout and form novel circuits to compensate for the loss of, and promote the return of, function. NVG-291 has been shown to promote clinically relevant improvements in a variety of preclinical models including spinal cord injury, peripheral nerve injury, stroke, and multiple sclerosis. Our goal is to explore NVG-291’s potential as a therapeutic intervention to facilitate recovery from neurodegenerative diseases.
Learning Objectives:
1. Describe how PTPσ and CSPGs inhibit functional recovery following nervous system damage.
2. Discuss potential applications of NVG-291 to promote regeneration, plasticity, and remyelination following nerve damage.
3. Analyze the rationale for NVG-291 and/or PTPσ modulation as a therapeutic intervention in degenerative diseases
Earn PACE Credits:
LabRoots on Social:
SnapChat: labroots_inc
Speaker Biography: Dr. Marc DePaul is the Director of Research at NervGen Pharma, a clinical stage biotech company dedicated to developing innovative treatments for nervous system repair. With over 11 years of experience in the biotech industry as a regenerative medicine scientist or consultant, his research strives to identify and develop therapeutics to enhance nervous system repair in disorders and injuries by promoting plasticity, regeneration, and remyelination. He received his Ph.D. in Neurosciences and studied as a post-doc at Case Western Reserve University, where he helped develop the technology NervGen’s platform is founded on.
Webinar: Keynote Presentation: Modulation of the CSPG Receptor PTPσ to Enhance Neurorepair
Webinar Abstract: There are currently no approved therapeutics to promote neural repair following central nervous system (CNS) damage. Groundbreaking studies in neurotrauma and disease found that a family of extracellular matrix (ECM) molecules known as chondroitin sulfate proteoglycans (CSPGs) are increased at sites of CNS damage and inhibit endogenous repair mechanisms. The inhibitory effects of CSPGs are mediated through protein tyrosine phosphatase sigma (PTPσ), a potent CSPG receptor. NervGen has developed a novel, first-in-class PTPσ modulator, known as NVG-291, for treatment of nervous system damage due to trauma or disease. NVG-291 is a systemically administered, blood-brain barrier penetrating peptide currently undergoing a Phase 1 trial in healthy volunteers to investigate safety, tolerability and pharmacokinetics.
NVG-291 modulates the cellular response and impact of CSPGs on neural repair, regeneration, remyelination, plasticity and other processes within the central and peripheral nervous systems. Following nervous system damage, CSPGs are upregulated within the forming scar around areas of damage, where they inhibit regeneration of injured axons. In non-damaged CNS tissue, CSPGs are a key component of the perineuronal net (PNN), an ECM structure surrounding synapses, where they help maintain synaptic connections by restricting axonal sprouting and plasticity. However, the presence of PNNs in an injured environment inhibits synaptic and axonal plasticity, which could otherwise facilitate recovery through the generation of novel circuits to compensate for injured or dysfunctional pathways. NVG-291 modulation of the CSPG receptor, PTPσ, promotes injured axons to regenerate through areas of damage, and non-injured axons away from damage to sprout and form novel circuits to compensate for the loss of, and promote the return of, function. NVG-291 has been shown to promote clinically relevant improvements in a variety of preclinical models including spinal cord injury, peripheral nerve injury, stroke, and multiple sclerosis. Our goal is to explore NVG-291’s potential as a therapeutic intervention to facilitate recovery from neurodegenerative diseases.
Learning Objectives:
1. Describe how PTPσ and CSPGs inhibit functional recovery following nervous system damage.
2. Discuss potential applications of NVG-291 to promote regeneration, plasticity, and remyelination following nerve damage.
3. Analyze the rationale for NVG-291 and/or PTPσ modulation as a therapeutic intervention in degenerative diseases
Earn PACE Credits:
LabRoots on Social:
SnapChat: labroots_inc
Комментарии
 0:59:51
0:59:51
 0:47:18
0:47:18
 1:10:44
1:10:44
 1:09:20
1:09:20
 0:55:18
0:55:18
 1:00:31
1:00:31
 0:48:44
0:48:44
 0:54:53
0:54:53
 0:58:35
0:58:35
 0:00:57
0:00:57
 0:00:37
0:00:37
 1:10:03
1:10:03
 0:45:56
0:45:56
 0:47:58
0:47:58
 0:00:36
0:00:36
 0:39:36
0:39:36
 0:46:36
0:46:36
 1:03:07
1:03:07
 0:52:50
0:52:50
 0:59:02
0:59:02
 1:01:30
1:01:30
 0:32:38
0:32:38
 0:58:14
0:58:14
 0:59:37
0:59:37