filmov
tv
Arrhenius Equation

Показать описание
Arrhenius! The founder of physical chemistry (you now know who to blame when you're cursing physical chemistry). This video looks into the Arrhenius equation, how you can use it to calculate activation energy and the effects on temperature and activation energy in the rate constant k. All useful and potentially a shed load of marks to gain!
The Arrhenius Equation
Arrhenius Equation
The Arrhenius Equation
14.4 Collision Theory and the Arrhenius Equation | General Chemistry
The Arrhenius equation | Kinetics | AP Chemistry | Khan Academy
Collision Theory - Arrhenius Equation & Activation Energy - Chemical Kinetics
The Arrhenius equation OCR A level Chemistry
Arrhenius Equation and Graph
Chemical Kinetics / L13 / numericals on first order kinetics and arrhenius equation
16.2 The Arrhenius equation (HL)
14.5 The Arrhenius Equation Example Problems
Arrhenius Equation (A-level IB Chemistry)
Arrhenius Equation Derivation
R2.2.12 The Arrhenius equation (HL)
Quick Revision - Arrhenius Equation
Arrhenius Equation for Reaction Rates
Arrhenius Equation | A level Chemistry | Question Walkthrough
arrhenius equation example
Energy Diagrams, Catalysts, and Reaction Mechanisms
Arrhenius equation / Activation energy / Temperature Dependence on Reaction rate / class12 chapter 4
Forms of the Arrhenius equation | Kinetics | Chemistry | Khan Academy
Rate Constant k and Arrhenius Equation made super simple MCAT
Rate Equations: Arrhenius Equation - Exam Question Walkthrough|AQA A Level Chemistry
Chemical Kinetics | class 12 (part 7.1) | Arrhenius Equation | Temp depend। Energy profile diagram
Комментарии
 0:05:41
0:05:41
 0:11:45
0:11:45
 0:06:17
0:06:17
 0:23:20
0:23:20
 0:09:24
0:09:24
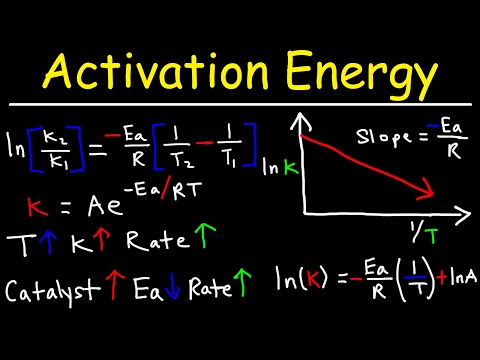 0:31:50
0:31:50
 0:06:57
0:06:57
 0:05:09
0:05:09
 1:02:49
1:02:49
 0:03:11
0:03:11
 0:05:02
0:05:02
 0:17:18
0:17:18
 0:04:04
0:04:04
 0:04:06
0:04:06
 0:04:41
0:04:41
 0:06:58
0:06:58
 0:19:23
0:19:23
 0:06:09
0:06:09
 0:05:23
0:05:23
 0:16:17
0:16:17
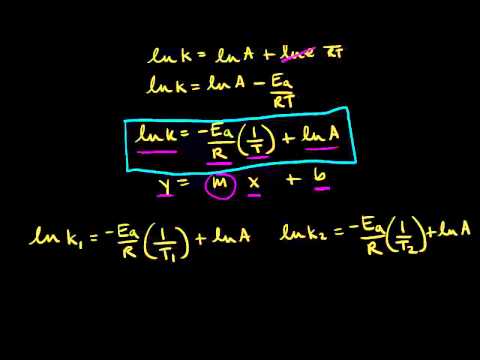 0:06:41
0:06:41
 0:07:35
0:07:35
 0:09:37
0:09:37
 0:12:41
0:12:41