filmov
tv
Alanine biochemistry - chirality, polarity, glucose-alanine cycle, beta-lactam antibiotics...
Показать описание
There’s a lot to love about ALANINE! It’s the smallest CHIRAL amino acid ever seen (mostly in the L-form!). Alanine is kinda the “generic” amino acid. It’s not the smallest (glycine beats it) but its methyl (CH₃) comes in 2nd. It isn’t very reactive, and is isn’t “essential” in the sense that we need to get it directly from our food but it IS very important. Just ask your muscles - which rely on the glucose-alanine cycle to remove nitrogen “waste” and get fresh sugar. ⠀
⠀
But today’s the spotlight’s on Alanine (abbreviated Ala, A), which is “coded for” by the RNA codon “words” starting with GC - so GCU, GCC, GCA, & GCG.
Alanine (Ala, A) has a methyl group (-CH₃) for its R. Atoms bond together by sharing electrons (negatively-charged subatomic particles that whizz around the atoms’ dense central nuclei where the positively-charged protons & neutral neutrons hang out). If they share fair, and there’s an equal number of protons & neutrons, the charges cancel out and the molecule is evenly charged everywhere. But if they don’t share fairly, the more electronegative (electron-hogging) atoms will pull electron density away from the less electronegative atoms, shifting the electron cloud and disrupting the even charge balance leading to a charge imbalance we call POLARITY.⠀
⠀
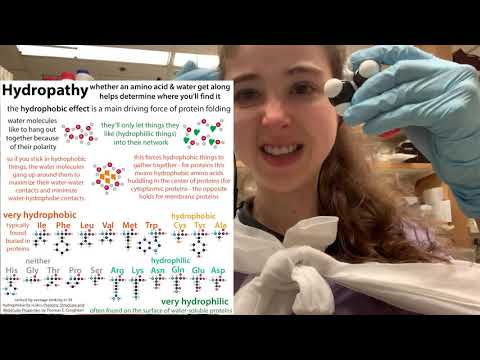
But C & H share pretty fairly and, as a result, CH₃ is considered “non polar.” Alanine may be non polar, but water definitely isn’t. It’s super polar (with O being the hog) and this is part of what makes it so great at dissolving things - opposite charges attract, so the partly negative O’s can hang out with partly or fully negative parts of other molecules, and the partly positive H’s can chill with partly or fully negative parts of other molecules. But methyl doesn’t offer these partial charge opportunities. So water wants to exclude it. This exclusion makes non polar things like methyl seem water-avoiding, so we call them HYDROPHOBIC. ⠀
⠀
Water excluding such things leads those things to gather in the center of the protein, away from water, and this “hydrophobic effect” is the main driver for protein folding. ⠀
Yesterday we looked at glycine, which is the simplest amino acid - its side chain is just an H. Now with alanine we’re dealing with a methyl group, CH₃. The addition of just 1 carbon and a couple hydrogens may seem small BUT has BIG effects. One of the main effects is that it makes Ala CHIRAL. By chiral I mean that Alanine (and all of the amino acids except for gylcine) has nonsuperimposable mirror images - like how the mirror image of your left hand looks like your right hand but they can’t fit into the same glove. ⠀
All the amino acids in proteins are in the L form. But the D form does have some uses. In fact, bacteria build cell walls out of peptidoglycan, which ends in D-Ala-D-Ala. The antibiotic ampicillin mimics this D-Ala-D-Ala, tricking the wall builder (transpeptidase) into trying to add it instead and getting stuck, leading to the cells being unable to build strong walls, so they burst & die. ⠀
⠀
Yesterday we looked at how glycine has very flexible backbone because its R’s so small, BUT CH₃, while still small, is bigger than H. So its backbone rotation’s limited by STERIC HINDRANCE (you can’t have neighboring atoms clash). This makes Ala more like other amino acids than glycine. ⠀
⠀
This structural “genericness” makes Ala a structural biologist’s friend. (note: “structural biology” is a field that investigates the link between the “shape” (form) and function of biochemical molecules. Think fork vs. spoon vs. knife but for proteins). It’s so “friend”-ly because you can stick in A as “placeholder” when you can’t see a protein region clearly in x-ray crystallography “pictures”. And its reactivity genericness makes it a protein biochemist’s friend because we can use it to test the importance of specific residues for a protein’s function - mutate that residue to Ala & look for an effect. Sometimes you know what specific residues you want to test, but other times you don’t know what will be important, so scientists sometimes do “alanine mutagenesis scans” where they basically try ‘em all out. ⠀
⠀
Ala may be “boring,” but it wins “Most likely to form α-helix!.” The α- helix is a common “secondary (2°) structure” (a motif within a protein’s overall “3° structure” that comes from backbone-backbone interactions)⠀
⠀
In addition to usefulness in the lab, alanine is super useful in our bodies through the GLUCOSE-ALANINE CYCLE: Alanine plays an important role in transferring extra nitrogen from peripheral tissues like muscles to your body’s main “detox” center, the liver. The nitrogen “piggy-backs” on the sugar breakdown product pyruvate which, once in the liver and freed of the nitrogen (which gets excreted as urea) it can be recycled into more sugar to be sent back to the muscles. ⠀
⠀
But today’s the spotlight’s on Alanine (abbreviated Ala, A), which is “coded for” by the RNA codon “words” starting with GC - so GCU, GCC, GCA, & GCG.
Alanine (Ala, A) has a methyl group (-CH₃) for its R. Atoms bond together by sharing electrons (negatively-charged subatomic particles that whizz around the atoms’ dense central nuclei where the positively-charged protons & neutral neutrons hang out). If they share fair, and there’s an equal number of protons & neutrons, the charges cancel out and the molecule is evenly charged everywhere. But if they don’t share fairly, the more electronegative (electron-hogging) atoms will pull electron density away from the less electronegative atoms, shifting the electron cloud and disrupting the even charge balance leading to a charge imbalance we call POLARITY.⠀
⠀
But C & H share pretty fairly and, as a result, CH₃ is considered “non polar.” Alanine may be non polar, but water definitely isn’t. It’s super polar (with O being the hog) and this is part of what makes it so great at dissolving things - opposite charges attract, so the partly negative O’s can hang out with partly or fully negative parts of other molecules, and the partly positive H’s can chill with partly or fully negative parts of other molecules. But methyl doesn’t offer these partial charge opportunities. So water wants to exclude it. This exclusion makes non polar things like methyl seem water-avoiding, so we call them HYDROPHOBIC. ⠀
⠀
Water excluding such things leads those things to gather in the center of the protein, away from water, and this “hydrophobic effect” is the main driver for protein folding. ⠀
Yesterday we looked at glycine, which is the simplest amino acid - its side chain is just an H. Now with alanine we’re dealing with a methyl group, CH₃. The addition of just 1 carbon and a couple hydrogens may seem small BUT has BIG effects. One of the main effects is that it makes Ala CHIRAL. By chiral I mean that Alanine (and all of the amino acids except for gylcine) has nonsuperimposable mirror images - like how the mirror image of your left hand looks like your right hand but they can’t fit into the same glove. ⠀
All the amino acids in proteins are in the L form. But the D form does have some uses. In fact, bacteria build cell walls out of peptidoglycan, which ends in D-Ala-D-Ala. The antibiotic ampicillin mimics this D-Ala-D-Ala, tricking the wall builder (transpeptidase) into trying to add it instead and getting stuck, leading to the cells being unable to build strong walls, so they burst & die. ⠀
⠀
Yesterday we looked at how glycine has very flexible backbone because its R’s so small, BUT CH₃, while still small, is bigger than H. So its backbone rotation’s limited by STERIC HINDRANCE (you can’t have neighboring atoms clash). This makes Ala more like other amino acids than glycine. ⠀
⠀
This structural “genericness” makes Ala a structural biologist’s friend. (note: “structural biology” is a field that investigates the link between the “shape” (form) and function of biochemical molecules. Think fork vs. spoon vs. knife but for proteins). It’s so “friend”-ly because you can stick in A as “placeholder” when you can’t see a protein region clearly in x-ray crystallography “pictures”. And its reactivity genericness makes it a protein biochemist’s friend because we can use it to test the importance of specific residues for a protein’s function - mutate that residue to Ala & look for an effect. Sometimes you know what specific residues you want to test, but other times you don’t know what will be important, so scientists sometimes do “alanine mutagenesis scans” where they basically try ‘em all out. ⠀
⠀
Ala may be “boring,” but it wins “Most likely to form α-helix!.” The α- helix is a common “secondary (2°) structure” (a motif within a protein’s overall “3° structure” that comes from backbone-backbone interactions)⠀
⠀
In addition to usefulness in the lab, alanine is super useful in our bodies through the GLUCOSE-ALANINE CYCLE: Alanine plays an important role in transferring extra nitrogen from peripheral tissues like muscles to your body’s main “detox” center, the liver. The nitrogen “piggy-backs” on the sugar breakdown product pyruvate which, once in the liver and freed of the nitrogen (which gets excreted as urea) it can be recycled into more sugar to be sent back to the muscles. ⠀
Комментарии
 0:35:04
0:35:04
 0:20:27
0:20:27
 0:11:39
0:11:39
 0:23:58
0:23:58
 0:11:34
0:11:34
 0:02:25
0:02:25
 0:23:34
0:23:34
 0:10:37
0:10:37
 0:13:58
0:13:58
 0:09:08
0:09:08
 0:31:02
0:31:02
 0:06:35
0:06:35
 0:08:09
0:08:09
 0:34:07
0:34:07
 0:34:01
0:34:01
 0:36:51
0:36:51
 0:33:54
0:33:54
 0:19:50
0:19:50
 0:08:56
0:08:56
 0:06:02
0:06:02
 0:26:38
0:26:38
 0:08:22
0:08:22
 0:11:28
0:11:28