filmov
tv
ALEKS: Drawing a box diagram of the electron configuration of an atom

Показать описание
ALEKS: Drawing a box diagram of the electron configuration of an atom
ALEKS - Drawing a box diagram of the electron configuration of an atom
ALEKS - Drawing a Box Diagram of the Electron Configuration of an Atom
Aleks Drawing a box diagram of the electron configuration of an atom
How to draw Electron-in-box diagrams Electronic Configurations? [GCE A Level Chemistry]
How to Add Orbital Boxes in ALEKS Tool
ALEKS: Drawing the MO energy diagram for a Period 2 homodiatom
Drawing a box diagram of the electron configuration of an atom
ALEKS: Interpreting the electron configuration of a neutral atom in noble gas notation
ALEKS: Drawing the Lewis dot diagram of a main group atom or common atomic ion
Orbital Box Diagrams
8.2c Drawing a box diagram of the electron configuration of an atom
ALEKS: Deducing valence electron configuration from trends in successive ionization energies
ALEKS - Drawing the MO energy diagram for a Period 2 homodiatom
Aleks Drawing the Lewis dot diagram of a main group atom or common atomic Ion example 1
ALEKS: Writing the electron configuration of a neutral atom with a and p electrons only
ALEKS: Sketching polarization induced by a nearby charge
CHE_106_Module_6_electron configuration_ALEKS
electron configuration and orbital box diagrams
ALEKS: Drawing the unit cell of a 2D lattice
ALEKS: Counting the electron shells in a neutral atom
ALEKS: Drawing Lewis structures for simple organic compounds
ALEKS - Reading a Periodic Table entry - Example 1
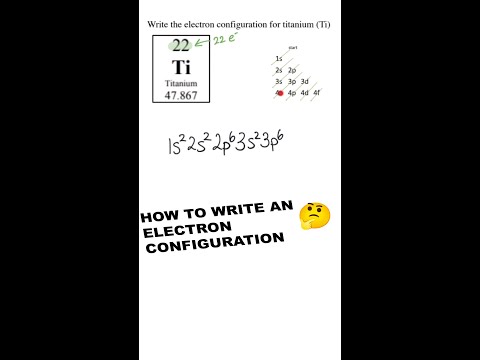
How to Write an Electron Configuration #chemistry #shorts #science #education #homework
Комментарии
 0:04:37
0:04:37
 0:06:49
0:06:49
 0:10:15
0:10:15
 0:05:50
0:05:50
 0:04:28
0:04:28
 0:01:14
0:01:14
 0:06:51
0:06:51
 0:08:04
0:08:04
 0:01:47
0:01:47
 0:07:20
0:07:20
 0:09:05
0:09:05
 0:03:44
0:03:44
 0:02:19
0:02:19
 0:15:02
0:15:02
 0:03:05
0:03:05
 0:02:26
0:02:26
 0:03:13
0:03:13
 0:04:22
0:04:22
 0:08:45
0:08:45
 0:04:04
0:04:04
 0:03:37
0:03:37
 0:08:00
0:08:00
 0:01:58
0:01:58
 0:01:00
0:01:00