filmov
tv
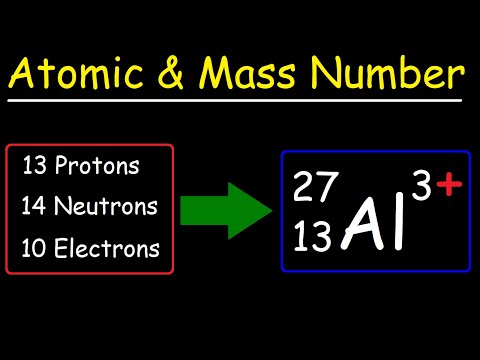
RAM VI Atomic Mass = Protons plus Neutrons

Показать описание
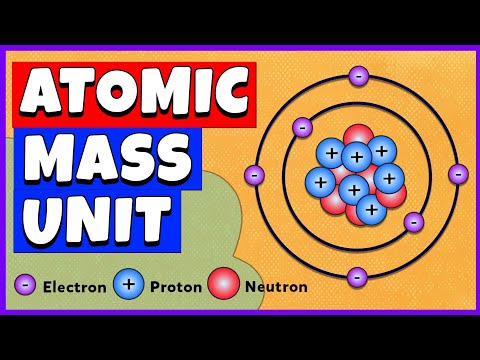
RAM = Relative Atomic Mass. This video explores the masses of the sub-atomic particles to show that only the protons and neutrons are responsible for 99.99% of the atom’s mass. This is because the electron’s mass is only 1/1836 th of their mass in comparison. On the other hand, the electron’s shells are responsible for the vast majority of the atom’s size.
The video also shows how we can work out how many neutrons are in a particular kind of atom, based on the difference between the Relative Atomic Mass and its Atomic Number; both of which are readily available from the Periodic Table. You will take a journey to seven different elements on the Periodic Table- hydrogen, helium, lithium, fluorine, scandium, chromium and zinc, and discover how to work out the number of neutrons that each has in its nucleus. You, too, can become a nuclear scientist!
One strange anomaly, however, gives a clue to the next level of understanding, coming up in the next video.
The video also shows how we can work out how many neutrons are in a particular kind of atom, based on the difference between the Relative Atomic Mass and its Atomic Number; both of which are readily available from the Periodic Table. You will take a journey to seven different elements on the Periodic Table- hydrogen, helium, lithium, fluorine, scandium, chromium and zinc, and discover how to work out the number of neutrons that each has in its nucleus. You, too, can become a nuclear scientist!
One strange anomaly, however, gives a clue to the next level of understanding, coming up in the next video.
 0:07:49
0:07:49
 0:03:48
0:03:48
 0:03:42
0:03:42
 0:04:16
0:04:16
 0:07:28
0:07:28
 0:11:41
0:11:41
 0:03:44
0:03:44
 0:03:59
0:03:59
 2:59:50
2:59:50
 0:04:14
0:04:14
 0:02:54
0:02:54
 0:10:18
0:10:18
 0:06:46
0:06:46
 0:04:24
0:04:24
 0:05:48
0:05:48
 0:13:12
0:13:12
 0:06:40
0:06:40
 0:11:53
0:11:53
 0:07:57
0:07:57
 0:04:29
0:04:29
 0:08:59
0:08:59
 0:07:20
0:07:20
 0:11:44
0:11:44
 0:05:16
0:05:16