filmov
tv
Atomic number || Mass number || Atomic mass || For Chemistry King

Показать описание
In this video you will come to know much about the atomic number, mass number & atomic mass.
DEFINITIONS:
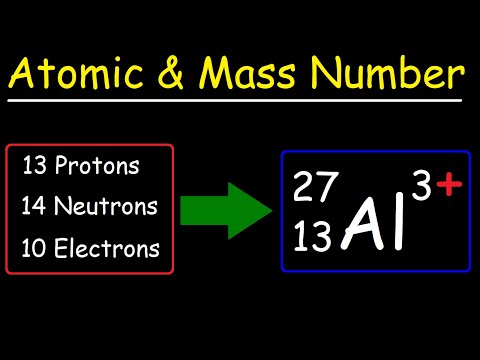
Atomic number : Atomic number of an element is equal to the number of protons present in one atom of that element.
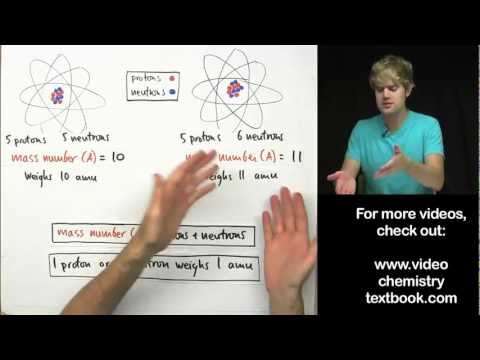
Mass number : Mass number of an element is the sum of the number of protons and neutrons present in the atom of that element.
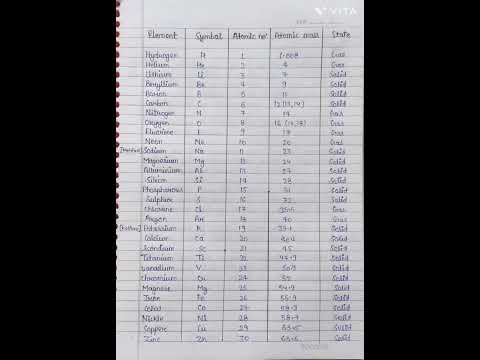
Atomic Mass : The atomic mass of an element is the average relative mass of its atoms as compared with the mass of carbon-12 isotope taken as 12 units.
History of atomic mass :
The reference which was chosen in the beginning was hydrogen atom because it was the lightest element. Its mass on the atomic mass scale was taken as 1. However, using hydrogen as the reference, the masses of atoms of other elements came out to be fractional. Hence, the reference was changed to oxygen taken as 16. This was selected because of the following two reasons :
1. Oxygen combined with most of the elements.
2. By comparing with oxygen taken as 16, the relative atomic masses of most of the elements were found to be whole numbers.
However, a difficulty arose when it was found that naturally occurring oxygen is a mixture of atoms of slightly different mass.
In 1961, IUPAC recommended the use of an isotope of carbon with mass number 12 as the standard reference for measuring atomic masses.
#AtomicNumber
#AtomicMass
#MassNumber
DEFINITIONS:
Atomic number : Atomic number of an element is equal to the number of protons present in one atom of that element.
Mass number : Mass number of an element is the sum of the number of protons and neutrons present in the atom of that element.
Atomic Mass : The atomic mass of an element is the average relative mass of its atoms as compared with the mass of carbon-12 isotope taken as 12 units.
History of atomic mass :
The reference which was chosen in the beginning was hydrogen atom because it was the lightest element. Its mass on the atomic mass scale was taken as 1. However, using hydrogen as the reference, the masses of atoms of other elements came out to be fractional. Hence, the reference was changed to oxygen taken as 16. This was selected because of the following two reasons :
1. Oxygen combined with most of the elements.
2. By comparing with oxygen taken as 16, the relative atomic masses of most of the elements were found to be whole numbers.
However, a difficulty arose when it was found that naturally occurring oxygen is a mixture of atoms of slightly different mass.
In 1961, IUPAC recommended the use of an isotope of carbon with mass number 12 as the standard reference for measuring atomic masses.
#AtomicNumber
#AtomicMass
#MassNumber
Комментарии
 0:03:23
0:03:23
 0:11:41
0:11:41
 0:03:50
0:03:50
 0:05:04
0:05:04
 0:02:24
0:02:24
 0:06:27
0:06:27
 0:05:33
0:05:33
 0:02:48
0:02:48
 0:03:11
0:03:11
 0:09:44
0:09:44
 0:02:05
0:02:05
 0:05:53
0:05:53
 0:08:57
0:08:57
 0:06:13
0:06:13
 0:08:26
0:08:26
 0:03:59
0:03:59
 0:13:12
0:13:12
 0:00:01
0:00:01
 0:11:46
0:11:46
 0:09:27
0:09:27
 0:04:35
0:04:35
 0:00:15
0:00:15
 0:02:40
0:02:40
 0:04:23
0:04:23