filmov
tv
Calculating the minimum temperature a reaction takes place spontaneously

Показать описание
Calculating the minimum temperature a reaction takes place spontaneously
Calculating the Temperature at Which a Reaction Becomes Spontaneous
How to use the Gibbs reaction to calculate the temperature at which a reaction is feasible
How to find the temperature when a reaction is spontaneous
Minimum Possible Temperature 🌡️🔥 #shorts #science
FINDING TEMPERATURE DIFFERENCE
Worked example: Determining the effect of temperature on thermodynamic favorability | Khan Academy
Effect of Temperature on Feasibility of Reactions
Live Tutorial Session 10 | Thermodynamics of Reactive Systems
How to determine your Temperature Minimum #andrewhuberman #neuroscience
How to find average/ mean temperature
Calculating ΔG°rxn Determining Effect of Temperature on Spontaneity
Calculate minimum Power - Heat & Temperature
Calculate Gibbs Free Energy; Calculate Temperature cut-off
Problem Table Approach to calculate utilities and pinch temperature - Lecture # 69
How Emissivity Can Impact Temperature Measurement with an Infrared Camera
Minimum temperature on planets.
How to Calculate Mean Kinetic Temperature (MKT) in Excel Sheet?
Solar Panel Power Temperature Coefficient
[JEE ADVANCED] A TRICK OF THERMODYNAMICS FOR FINDING MAXIMUM AND MINIMUM TEMPERATURE IN CYCLE
Mechanical Engineering Thermodynamics | Temperature and how to use it in thermodynamic calculations
How Temperature Varies During Day and Night | DIU
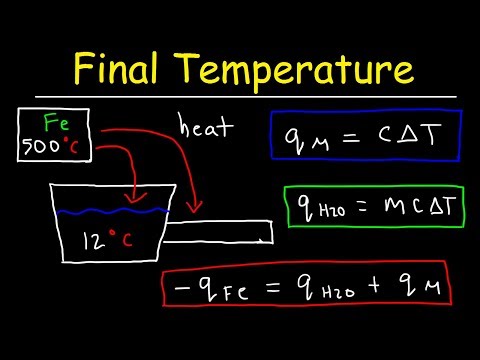
Final Temperature Calorimetry Practice Problems - Chemistry
Converting Between Temperature Scales (Celsius, Fahrenheit, and Kelvin)
Комментарии
 0:05:28
0:05:28
 0:03:51
0:03:51
 0:03:26
0:03:26
 0:05:29
0:05:29
 0:00:45
0:00:45
 0:14:04
0:14:04
 0:06:47
0:06:47
 0:05:24
0:05:24
 2:00:51
2:00:51
 0:00:35
0:00:35
 0:02:54
0:02:54
 0:03:21
0:03:21
 0:05:11
0:05:11
 0:12:27
0:12:27
 0:14:15
0:14:15
 0:04:35
0:04:35
 0:00:58
0:00:58
 0:01:21
0:01:21
 0:02:33
0:02:33
![[JEE ADVANCED] A](https://i.ytimg.com/vi/NN13d-1SSHs/hqdefault.jpg) 0:04:15
0:04:15
 0:06:10
0:06:10
 0:00:27
0:00:27
 0:18:16
0:18:16
 0:06:18
0:06:18