filmov
tv
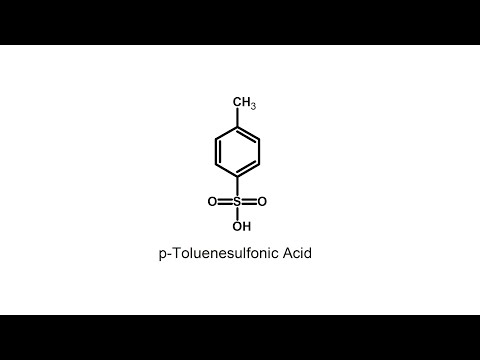
Make p-Toluenesulfonic Acid

Показать описание
In this video we make p-Toluenesulfonic acid from sulfuric acid based drain cleaner and paint thinner containing toluene.
Donate to NurdRage!
Through Bitcoin: 1NurdRAge7PNR4ULrbrpcYvc9RC4LDp9pS
Use the discount code "nurdrage" for a 5% discount.
Donate to NurdRage!
Through Bitcoin: 1NurdRAge7PNR4ULrbrpcYvc9RC4LDp9pS
Use the discount code "nurdrage" for a 5% discount.
Make p-Toluenesulfonic Acid
Sulfonation: p-Toluenesulfonic Acid
p-Toluenesulfonic Acid Footage
Preparation of toluene and (de)sulfonation in detail
Toluenesulfonic acid Meaning
European P Toluenesulfonic Acid Industry 2016 Deep Market Research Report
Toluene Sulfonic Acid Manufacturing Process, Machinery Requirements and Project Report
Global P Toluenesulfonic Acid Market Insights and Forecast to 2027
Synthesis of p-Chlorotoluene
SYNTHESIS OF CARBOXY METHYLBENZENE (toluic acid) PART 4
Making Tropic Acid
Sulfonic Acids:Introduction and Preparation.
SYNTHESIS OF CARBOXY METHYLBENZENE (toluic acid) PART 5
acrylic acid making process(2)
Making Piperidine to piss off my FBI Agent
Alcohols are converted to tosylates by treatment with p-toluene sulfonyl chloride (TsCl) in the p...
Make Hydrobromic Acid Revisited 2018
DANGEROUS reaction of ACID and GLOVES
SULPHONIC ACID - LABSA 5 t/h
Is Ruthenium Jewelry Dangerous?
GCMS batch file setup
Making skatole - The essence of poop
Sulfanilic acid : Organic synthesis
p-xylene reaction
Комментарии
 0:13:57
0:13:57
 0:19:30
0:19:30
 0:00:50
0:00:50
 0:08:09
0:08:09
 0:00:38
0:00:38
 0:00:31
0:00:31
 0:01:06
0:01:06
 0:00:41
0:00:41
 0:06:14
0:06:14
 0:14:32
0:14:32
 0:07:54
0:07:54
 0:10:23
0:10:23
 0:14:27
0:14:27
 0:00:16
0:00:16
 0:06:54
0:06:54
 0:04:15
0:04:15
 0:10:08
0:10:08
 0:04:53
0:04:53
 0:00:48
0:00:48
 0:04:42
0:04:42
 0:10:42
0:10:42
 0:12:44
0:12:44
 0:05:28
0:05:28
 0:00:23
0:00:23