filmov
tv
Expedited US FDA Development and Approval Programs for Serious Conditions

Показать описание
Successfully navigate US FDA’s expedited programs for development of products for serious conditions: Understand the data expectations, planning and timing skills, and authoring strategies to optimize the chance of securing an expedited program designation.
 0:56:50
0:56:50
 0:04:44
0:04:44
 0:55:45
0:55:45
 0:02:55
0:02:55
 0:02:26
0:02:26
 0:00:07
0:00:07
 0:03:44
0:03:44
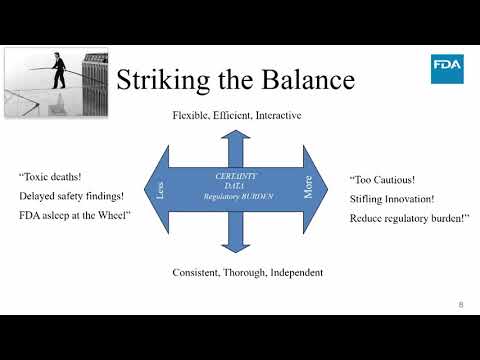 0:53:28
0:53:28
 0:35:01
0:35:01
 0:54:56
0:54:56
 0:04:22
0:04:22
 0:02:43
0:02:43
 0:09:16
0:09:16
 0:54:56
0:54:56
 0:26:13
0:26:13
 0:35:58
0:35:58
 0:02:10
0:02:10
 0:07:38
0:07:38
 0:06:01
0:06:01
 0:02:09
0:02:09
 0:00:37
0:00:37
 0:01:05
0:01:05
 0:58:33
0:58:33
 0:01:53
0:01:53