filmov
tv
Photoelectric Effect, Work Function, Threshold Frequency, Wavelength, Speed & Kinetic Energy, Electr
Показать описание
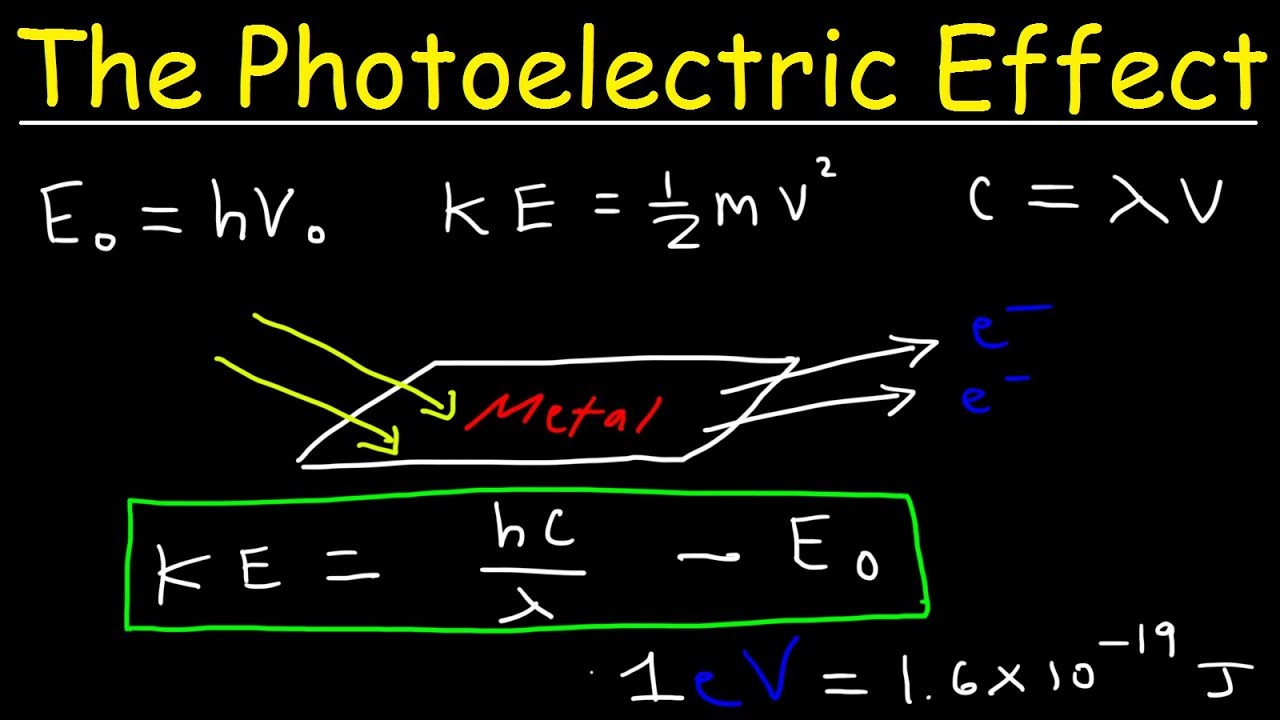
This chemistry video tutorial explains how the photoelectric effect works. It also explains how to use the work function of metals to calculate the threshold frequency and maximum wavelength of light necessary to eject an electron off the surface of an active metal. In addition, it explains how to calculate the speed of an electron as well as the kinetic energy of an electron in electron volts. You need to know how to convert electron volts to joules and vice versa. This video provides all of the formulas and equations needed including plenty of practice problems and examples.
Speed of Light, Frequency, Wavelength:
Photon Energy:
The Photoelectric Effect:
De Broglie Wavelength:
The Bohr Model of Hydrogen:
Heisenberg's Uncertainty Principle:
________________________________
Intro to Quantum Numbers:
Orbitals & Atomic Energy Levels:
Maximum Number of Electrons:
Intro to Electron Configuration:
Electron Configuration Exceptions:
Noble Gas Notation:
Electron Configuration of Ions:
_______________________________
Orbital Diagrams:
Paired & Unpaired Electrons:
Aufbau's Principle & Hund's Rule:
Paramagnetic & Diamagnetic Elements:
Valence Electrons & Periodic Table:
Effective Nuclear Charge:
_________________________________
Slater's Rule:
How To Identify The Element:
Quantum Numbers - Mega Review:
Quantum Numbers - Practice Test:
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Speed of Light, Frequency, Wavelength:
Photon Energy:
The Photoelectric Effect:
De Broglie Wavelength:
The Bohr Model of Hydrogen:
Heisenberg's Uncertainty Principle:
________________________________
Intro to Quantum Numbers:
Orbitals & Atomic Energy Levels:
Maximum Number of Electrons:
Intro to Electron Configuration:
Electron Configuration Exceptions:
Noble Gas Notation:
Electron Configuration of Ions:
_______________________________
Orbital Diagrams:
Paired & Unpaired Electrons:
Aufbau's Principle & Hund's Rule:
Paramagnetic & Diamagnetic Elements:
Valence Electrons & Periodic Table:
Effective Nuclear Charge:
_________________________________
Slater's Rule:
How To Identify The Element:
Quantum Numbers - Mega Review:
Quantum Numbers - Practice Test:
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Комментарии
 0:22:57
0:22:57
 0:03:12
0:03:12
 0:04:23
0:04:23
 0:09:39
0:09:39
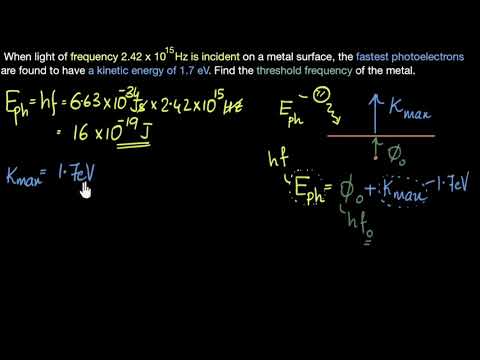 0:08:49
0:08:49
 0:22:00
0:22:00
 0:02:31
0:02:31
 0:13:23
0:13:23
 0:10:39
0:10:39
 0:07:42
0:07:42
 0:12:09
0:12:09
 0:17:13
0:17:13
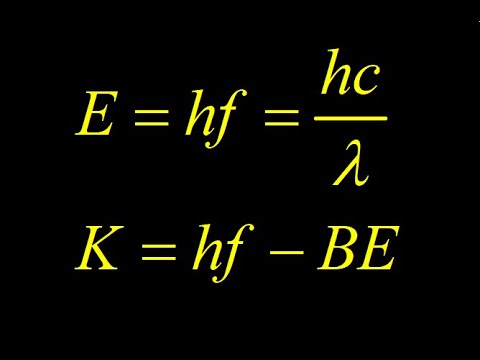 0:04:02
0:04:02
 0:00:58
0:00:58
 0:48:48
0:48:48
 0:10:26
0:10:26
 0:13:32
0:13:32
 0:10:16
0:10:16
 0:00:27
0:00:27
 0:05:58
0:05:58
 0:09:34
0:09:34
 0:05:55
0:05:55
 0:09:52
0:09:52
 0:00:57
0:00:57