filmov
tv
Drawing Lewis Dot Structures 4 of 10 | Molecular Geometry | www.whitwellhigh.com

Показать описание
Lewis Diagrams Made Easy: How to Draw Lewis Dot Structures
Lewis Dot Structures
How To Draw Lewis Structures
How to Draw the Lewis Dot Structure for SiO4 4-: Silicate ion
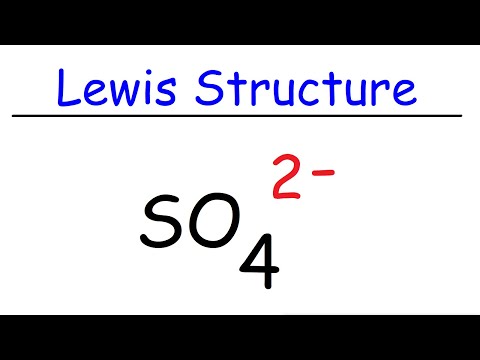
How To Draw The Lewis Structure of SO4 2- (Sulfate Ion)
PO4 3- Lewis Structure: How to Draw the Lewis Structure for PO4 3-
Lewis Structures Made Easy: Examples and Tricks for Drawing Lewis Dot Diagrams of Molecules
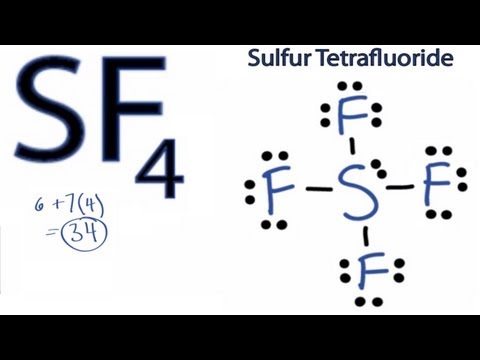
SF4 Lewis Structure: How to Draw the Lewis Structure for SF4
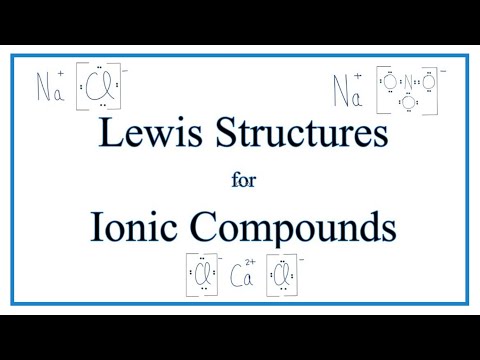
How to Draw Lewis Dot Structures for Ionic Compounds/Bonds
How to Draw Lewis Structures: Five Easy Steps
How to Draw The Lewis Structure for CF4 (Carbon Tetrafluoride)
How to Draw the Lewis Dot Structure for SO4 2- (Sulfate ion)
How to Draw the Lewis Structure for the Sulfate Ion
BF4- Lewis Structure - How to Draw the Lewis Structure for BF4-
P4 Lewis Structure: How to Draw the Lewis Structure for P4
CBr4 Lewis Structure: How to Draw the Lewis Structure for CBr4
Drawing Lewis diagrams | AP Chemistry | Khan Academy
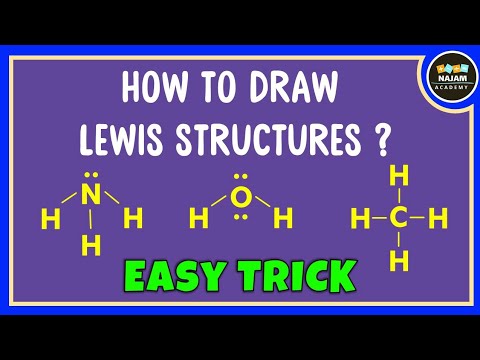
How to draw Lewis structure of any compound? Easy Trick
Lewis Structures for Covalent Molecules: Step-by-Step
PO4 3- Lewis Structure - The Phosphate Ion
How to Draw Lewis Structures, The Octet Rule and Exceptions | Study Chemistry With Us
Lewis Structure | Trick to Draw Lewis Dot Structures
BH4- Lewis Structure: How to Draw the Lewis Structure for the BH4 -
How to Draw the Lewis Dot Structure for BrF4-
Комментарии
 0:07:26
0:07:26
 0:04:41
0:04:41
 0:11:50
0:11:50
 0:01:32
0:01:32
 0:06:26
0:06:26
 0:03:12
0:03:12
 0:11:57
0:11:57
 0:01:49
0:01:49
 0:05:42
0:05:42
 0:05:57
0:05:57
 0:01:22
0:01:22
 0:04:30
0:04:30
 0:04:19
0:04:19
 0:01:11
0:01:11
 0:01:46
0:01:46
 0:01:00
0:01:00
 0:07:08
0:07:08
 0:06:19
0:06:19
 0:10:29
0:10:29
 0:07:24
0:07:24
 0:36:27
0:36:27
 0:22:42
0:22:42
 0:01:15
0:01:15
 0:01:46
0:01:46