filmov
tv
Calculate Molecular Formula From Empirical - Practice - 2
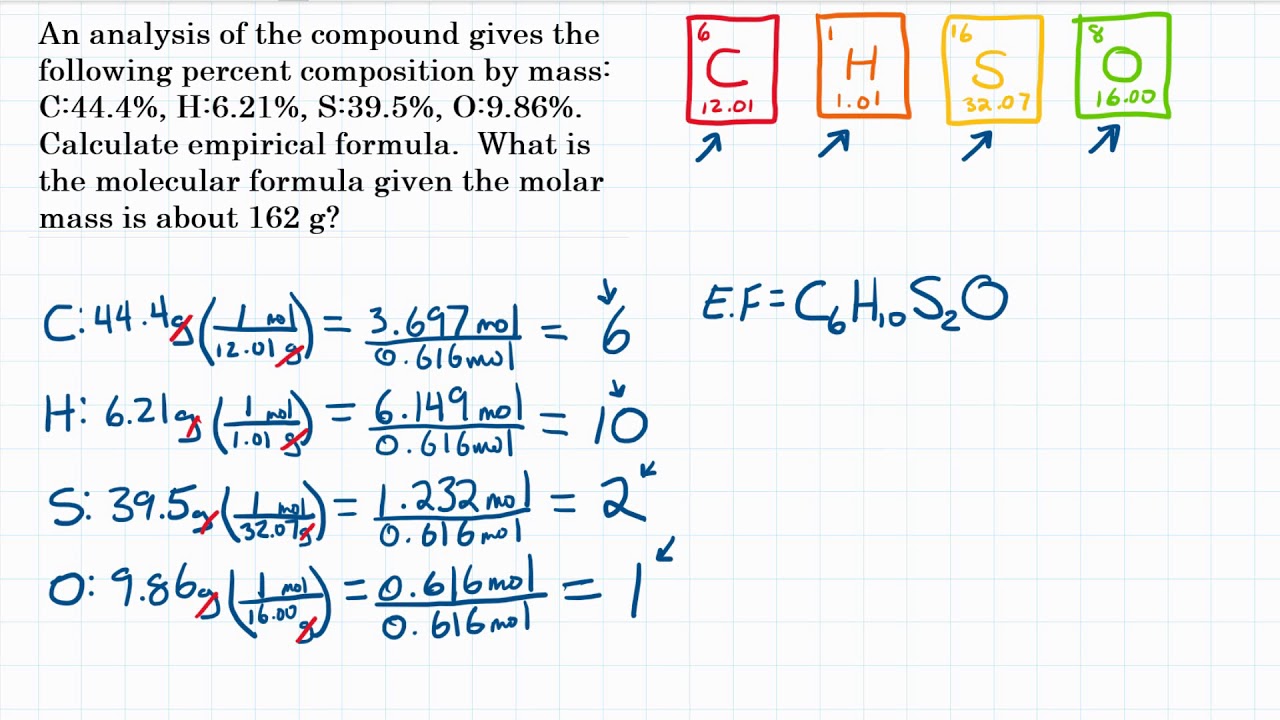
Показать описание
Allicin is the compound responsible for the characteristic smell of garlic. An analysis of the compound gives the following percent composition by mass: C:44.4 percent; H: 6.21 percent; S: 39.5 percent; O: 9.86 percent. Calculate its empirical formula. What is its molecular formula given that its molar mass is about 162 g?
Teachers Pay Teachers Practice Worksheets:
Molecular Formula Practice 1.0:
Answer Key 2.0:
Question is credited to Chang Chemistry 10th Edition Question 3.43
Teachers Pay Teachers Practice Worksheets:
Molecular Formula Practice 1.0:
Answer Key 2.0:
Question is credited to Chang Chemistry 10th Edition Question 3.43
Calculating Molecular Formula from Empirical Formula
Empirical Formula & Molecular Formula Determination From Percent Composition
Calculating Molecular Formulas Step by Step | How to Pass Chemistry
Calculating Molecular Formula from Empirical Formula
Practice Problem: Empirical and Molecular Formulas
Empirical Formula and Molecular Formula Introduction
How to calculate empirical formula given a molecular formula
How to Find the Molecular formula from the Empirical Formula and Molar Mass
CBSE 11 Chemistry | Some Basic Concepts Of Chemistry | NEET Chemistry Previous Year Questions
Molecular Formula from Empirical Formula & Molar Mass
Empirical to Molecular Formula - 3 Simple Steps - How to Find Molecular from Empirical
Calculating the molecular formula given empirical formula and molar mass
Finding and Calculating an Empirical Formula of a Compound | How to Pass Chemistry
⚗️ Calculating a Molecular Formula from an Empirical Formula and Molar Mass (Part 1)
Empirical Formula and Molecular Formula | Basic Concept | Numerical Problems
Introduction to Combustion Analysis, Empirical Formula & Molecular Formula Problems
Empirical and Molecular Formula from Percent Composition (No. 1)
Writing Empirical Formula Practice Problems
Empirical and Molecular Formula Calculations
How to calculate Empirical Formula? 3 Easy Steps
How To Find Empirical Formula And Molecular Formula Of A Compound? || With Example|| Chemistry ||
Determining Empirical and Molecular Formulas - Chemistry Tutorial
Calculate Empirical and Molecular Formulas (Tagalog Version)
How to calculate the Empirical and Molecular Formula of Compounds for 2025 JAMB tutorial
Комментарии
 0:09:09
0:09:09
 0:11:00
0:11:00
 0:04:26
0:04:26
 0:06:06
0:06:06
 0:05:53
0:05:53
 0:08:31
0:08:31
 0:03:05
0:03:05
 0:04:47
0:04:47
 0:54:05
0:54:05
 0:07:25
0:07:25
 0:04:00
0:04:00
 0:01:28
0:01:28
 0:02:52
0:02:52
 0:04:29
0:04:29
 0:13:30
0:13:30
 0:16:49
0:16:49
 0:08:47
0:08:47
 0:06:09
0:06:09
 0:05:33
0:05:33
 0:05:01
0:05:01
 0:01:01
0:01:01
 0:08:10
0:08:10
 0:20:16
0:20:16
 0:13:40
0:13:40